Updated WHO severe COVID treatment guidelines bit.ly/3rhGgKW
Recommended
🟢Steroid (#Dexamethasone)
🟢IL6 blocker (#Toci) or 🟢JAKi (#Baricitinib)
🟠±mAb in seronegative people
NOT recommended
🔴#Hydroxychloroquine
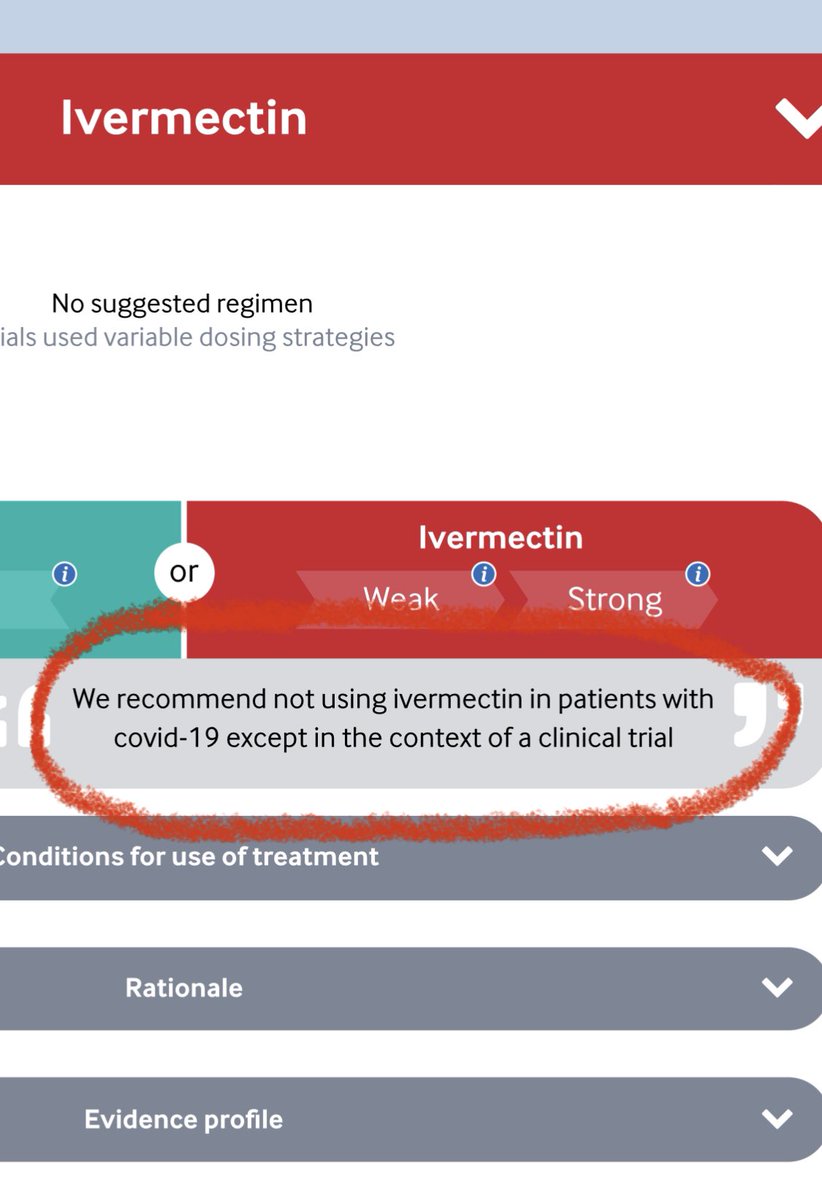
🔴#ivermectin
🔴#remdesivir
Lots to discuss, a🧵
1/


Recommended
🟢Steroid (#Dexamethasone)
🟢IL6 blocker (#Toci) or 🟢JAKi (#Baricitinib)
🟠±mAb in seronegative people
NOT recommended
🔴#Hydroxychloroquine
🔴#ivermectin
🔴#remdesivir
Lots to discuss, a🧵
1/


Remdesivir (RDV) is in the “We suggest no remdesivir” category.
At some level, this isn’t too surprising & is old news.
Despite initial hype, RDV never moved the needle much on patient centered outcomes (risk of mortality or requiring IMV) & many of us had stopped using it.
2/
At some level, this isn’t too surprising & is old news.
Despite initial hype, RDV never moved the needle much on patient centered outcomes (risk of mortality or requiring IMV) & many of us had stopped using it.
2/

In #ACTT1 RDV did improve outcomes on an ordinal scale, but the effect was modest. It shortened time to clinical improvement but not hospital LOS (patients stayed in the hospital longer to receive it).
RDV did NOT improve mortality or risk of IMV.
ncbi.nlm.nih.gov/pubmed/32445440
3/


RDV did NOT improve mortality or risk of IMV.
ncbi.nlm.nih.gov/pubmed/32445440
3/



In #DisCoVeRy, an n=857 adaptive open label trial in Europe (🇫🇷 🇧🇪 🇦🇹 🇵🇹 🇱🇺), RDV had no clinical benefit in terms of mortality, risk of mechanical ventilation.
Unlike ACTT-A, no major differences in ordinal scale were seen.
pubmed.ncbi.nlm.nih.gov/34534511/
4/
Unlike ACTT-A, no major differences in ordinal scale were seen.
pubmed.ncbi.nlm.nih.gov/34534511/
4/

In #Solidarity, a very large (n=5451) global (🌍) open label RCT of repurposed drugs run by the WHO, RDV again had no clinical benefit in terms of hospital mortality or need for IMV.
(Solidarity study also conclusively disproved benefit from HCQ)
5/
ncbi.nlm.nih.gov/pubmed/33264556

(Solidarity study also conclusively disproved benefit from HCQ)
5/
ncbi.nlm.nih.gov/pubmed/33264556


Likewise, I think almost everyone is on the steroids PLUS train, where dexamethasone is combined with another immune modulator, either a JAKinhibitor (Bariticinib) or an IL-6 blocker.
6/
6/
There’s strong evidence for steroids in COVID as well as fairly strong evidence of Bari & Toci too.
See prior threads
Dex
Toci
Bari
7/
See prior threads
Dex
https://twitter.com/nickmmark/status/1275187455117848576?s=20
Toci
https://twitter.com/nickmmark/status/1359964191306567680?s=20
Bari
https://twitter.com/nickmmark/status/1433213943275679748?s=20
7/
I think one big area of uncertainty is which drug to combine with steroids and in whom.
- Bari has the advantage of being a pill & slightly cheaper than Toci
- The effect size for Bari also appears to be larger: OR for mortality is in the 0.6 range compared to 0.8 for toci
8/
- Bari has the advantage of being a pill & slightly cheaper than Toci
- The effect size for Bari also appears to be larger: OR for mortality is in the 0.6 range compared to 0.8 for toci
8/
Importantly, while we can use different IL-6 receptor blockers (e.g. tocalizumab or sarilumab) we should NOT generalize using the class of JAK inhibitors:
Specifically, the WHO recommends using Baricitinib (Bari) but suggests NOT using Tofacitinib (Tofa) & Ruxolitinib (Rux).
9/
Specifically, the WHO recommends using Baricitinib (Bari) but suggests NOT using Tofacitinib (Tofa) & Ruxolitinib (Rux).
9/

I agree with this. We should NOT view the different JAK inhibitors as fungible.
They have key differences in the kinome profile & different immune modulatory effects. While the data for Bari looks very good, the data for Tofacitinib & especially Ruxolitinib is less impressive
10/


They have key differences in the kinome profile & different immune modulatory effects. While the data for Bari looks very good, the data for Tofacitinib & especially Ruxolitinib is less impressive
10/



More interesting is the WHO recommendation about inpatient use of monoclonal Abs.
Previously the best (and only) data for mAbs was in outpatients to prevent the composite outcome of death & hospitalization. In fact, the EUA for all approved mAbs only covers outpatient use.
11/


Previously the best (and only) data for mAbs was in outpatients to prevent the composite outcome of death & hospitalization. In fact, the EUA for all approved mAbs only covers outpatient use.
11/



The change is driven by a large mortality reduction seen in RECOVERY.
This open label trial randomized n=9785 to REGEN-COV (casirivimab & imdevimab) vs usual care. They found a 6% reduction in mortality & 7% in IMV but ONLY in seronegative people.
medrxiv.org/content/10.110…
12/

This open label trial randomized n=9785 to REGEN-COV (casirivimab & imdevimab) vs usual care. They found a 6% reduction in mortality & 7% in IMV but ONLY in seronegative people.
medrxiv.org/content/10.110…
12/


The 6% absolute mortality reduction is pretty impressive tbh.
I suspect the key was a higher dose of mAb (4g casirivimab + 4g imdevimab), given early (mean 7 days since sx, 1 day since admission) to high risk patients (those who are seronegative)
A couple questions linger
13/
I suspect the key was a higher dose of mAb (4g casirivimab + 4g imdevimab), given early (mean 7 days since sx, 1 day since admission) to high risk patients (those who are seronegative)
A couple questions linger
13/
Are seronegative people “non-responders” (unable to make IgG against spike protein) or are they just earlier in their illness? (Haven’t made IgG yet)
How does vaccine timing factor in?
How to operationalize rapid measurement of anti-spike IgG? (This is the most crucial one)
14/

How does vaccine timing factor in?
How to operationalize rapid measurement of anti-spike IgG? (This is the most crucial one)
14/


Since mAbs are scarce, is it better to use them inpatient (to prevent IMV & mortality) or outpatient to prevent hospitalization (& potentially avert collapse of the health system)?
A tough health policy question.
Finally, does REGEN-COV even matter with omicron now dominant?
15/
A tough health policy question.
Finally, does REGEN-COV even matter with omicron now dominant?
15/
I’ve written extensively about how the data from in vitro, observational, & interventional trials doesn’t support the use of ivermectin in COVID.
Not even one (non fraudulent) RCT shows a mortality benefit.
EVERY high quality RCT has been negative.
17/
Not even one (non fraudulent) RCT shows a mortality benefit.
EVERY high quality RCT has been negative.
https://twitter.com/nickmmark/status/1443664149209223188?s=21
17/
If you prefer watching video to reading text, here’s a grand rounds lecture I gave about debunking IVERMANIA.
18/
18/
Bottom line: There are some areas of uncertainty (testing for seronegativity) & a few glaring omissions (fluvoxamine) but overall these are good guidelines based on solid evidence IMO. I’m curious to see if NIH changes their guidelines to align to this (RDV, REGEN-COV)
19/19
19/19
• • •
Missing some Tweet in this thread? You can try to
force a refresh