In recognition of the news that $SGMO has dosed the first human with engineered Tregs for renal transplant patients, here is a thread for anyone interested.
1/
1/
Before we look at some previous $SGMO disclosures it's useful to note that this has become a very active investment sector in the past 2 years. The largest splash was made when $MRK acquired Pandion in Feb 2021 for $1.85b or 3x the market cap. Pandion=Talon effector cells
2/
2/

OrcaBio announced a raise of $192m in 2020
GentiBio received $157m in Aug 2021
Quell raised $156m in Nov 2021 after they received CTA approval for a liver transplant treatment similar to $SGMO
3/


GentiBio received $157m in Aug 2021
Quell raised $156m in Nov 2021 after they received CTA approval for a liver transplant treatment similar to $SGMO
3/



Some others include AZTherapies, Cellenkos, Coya and Regimmune
T regulatory and effector cell activity has been nothing short of robust over the past 2 yrs
5/



T regulatory and effector cell activity has been nothing short of robust over the past 2 yrs
5/




Some useful info was presented by $SGMO on R&D day and subsequent which I'll include here. Much of this will apply specifically to Quell and others as well
6/
6/
It's useful to note that engineered regulatory T cells:
- are targeted at larger populations
- have real potential for LT remission
- can promote tissue regeneration
7/

- are targeted at larger populations
- have real potential for LT remission
- can promote tissue regeneration
7/


A large body of published research provides evidence that regulatory cell dysfunction is a contributing factor in autoimmune/inflammatory diseases. Importantly this is no singular MoA providing a larger targeting molecular pathway
8/

8/


Some referenced work by Dr Powrie at Oxford shows in a mouse IBD model that Tregs reversed the disease which was induced prior to treatment.
9/

9/


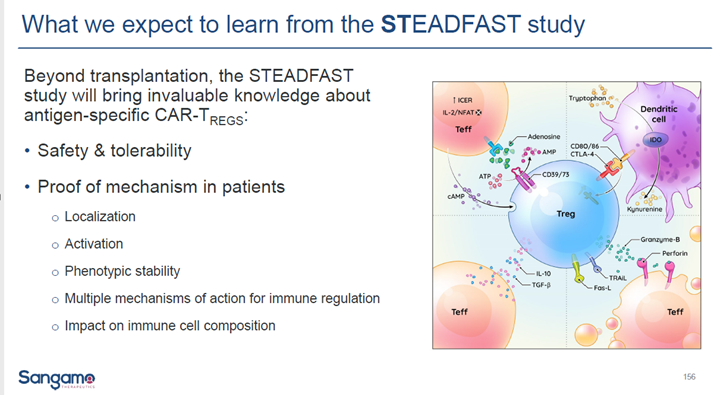
$SGMO TX200 mice study shows CAR drives Tregs to accumulate is the targeted tissue via antigen recognition and expand leading to conclusion that off target risk is mitigated by approach
10/
10/

The HLA-A2 mismatched size is 21-26% of the renal transplant market. The $SGMO #Steadfast study is hoped to provide this group the opportunity to stop taking anti-rejection drugs
12/


12/



This study is actually a gateway. This is autologous with a follow on program in preclinical development for allogeneic. Also version 2.0 of engineered CAR-Treg cell therapy is in $SGMO development and the company has process dev and in house manufacturing ready.
13/


13/



Little is know still about the CAR construct that was developed. $SGMO Dr Fontenot has hinted it grew out of the learnings from oncology CT and then significantly modified with addl info to be defined at a future date.
14/

14/


In summary this is an exciting new clinical step that opens the door to the future of CAR Tregs. Keep an eye out for both Steadfast and Liberate from Quell. Fingers crossed for both!
15/

15/


CAR Treg published commentary on $SGMO Steadfast trial protocol
- Includes auto stop of dose escalation for tox
- Intend to attempt taper of immunosuppression regimen b4 and after 16 wk biopsy
16/
kireports.org/article/S2468-…
- Includes auto stop of dose escalation for tox
- Intend to attempt taper of immunosuppression regimen b4 and after 16 wk biopsy
16/
kireports.org/article/S2468-…
Quell Liberate trial also anticipates early tapering of immunosuppression drugs and primary safety measure in 28 days
17/
17/

Coya ALS Treg pub
nn.neurology.org/content/9/6/e2…
nn.neurology.org/content/9/6/e2…
Sonoma using some of the invested cash to open R&D and Mfg facility in Seattle
biopharmadive.com/news/sonoma-tr…
biopharmadive.com/news/sonoma-tr…
Coya ALS P2a pub: 6 of 8 patients slowed/stopped progression
coyatherapeutics.com/news/10-press-…
coyatherapeutics.com/news/10-press-…
GentiBio preclinical data on Treg engineering
gentibio.com/press_releases…
gentibio.com/press_releases…
OrcBio adding 100k sq ft mfg in Sacramento for Tregs
orcabio.com/2022-09-08/
orcabio.com/2022-09-08/
Quell partnered with Ncardia spinoff Cellistic to create an allo treg platform based upon iPSCs
cellistic.com/insights/quell…
cellistic.com/insights/quell…
Updated recap of Treg group
- Abata ABA-101 progressed to IND enabling
- #COYA IPO
- Cugene lead $ABBV to clinic w/healthy vol
- gentibio $BMY collab
- Kyverna lead for Lupus IND cleared
- Quell QEL-001 lead not yet in clinic
$SGMO TX200 dosing slow


- Abata ABA-101 progressed to IND enabling
- #COYA IPO
- Cugene lead $ABBV to clinic w/healthy vol
- gentibio $BMY collab
- Kyverna lead for Lupus IND cleared
- Quell QEL-001 lead not yet in clinic
$SGMO TX200 dosing slow



@threadreaderapp unroll please
• • •
Missing some Tweet in this thread? You can try to
force a refresh