Please read our latest review by @jan_choutka et al. on “Unexplained post-acute infection syndromes”.
What a privilege to work with Jan Choutka, who is an #MECFS patient, expert and advocate. Grateful to @mhornig on her expertise/insights 🙏🏼 (1/)
nature.com/articles/s4159…
What a privilege to work with Jan Choutka, who is an #MECFS patient, expert and advocate. Grateful to @mhornig on her expertise/insights 🙏🏼 (1/)
nature.com/articles/s4159…
With millions of #longCOVID patients, it is becoming better known that even a mild infection can lead to longterm debilitating health problems. SARS-CoV-2 joins the long list of other pathogens that cause post-acute infection syndrome (PAIS). (2/) 

What are some common and distinct symptoms associated with PAIS? Strikingly, there are a number of shared symptoms such as excertion intolerance, fatigue, pain, neurological symptoms..etc. Others are more unique to the pathogen that triggered the disease. (3/) 

What percentage of people who are infected develop PAIS? Taking an example of mononucleosis associated with Epstein-Barr virus (EBV) infection, #MECFS persisted in ~4% of the participants who continue to experience debilitating symptoms at 2-year follow-up 👇🏽 (4/) 

Significant percentages of those infected with #SARSCoV2 report overall #longCOVID (blue) or activity-limiting long COVID (red). Over time, there is some gradual decline but persistence of disease ~6 months since the infection. Source: Office for National Statistics (ONS). (5/) 

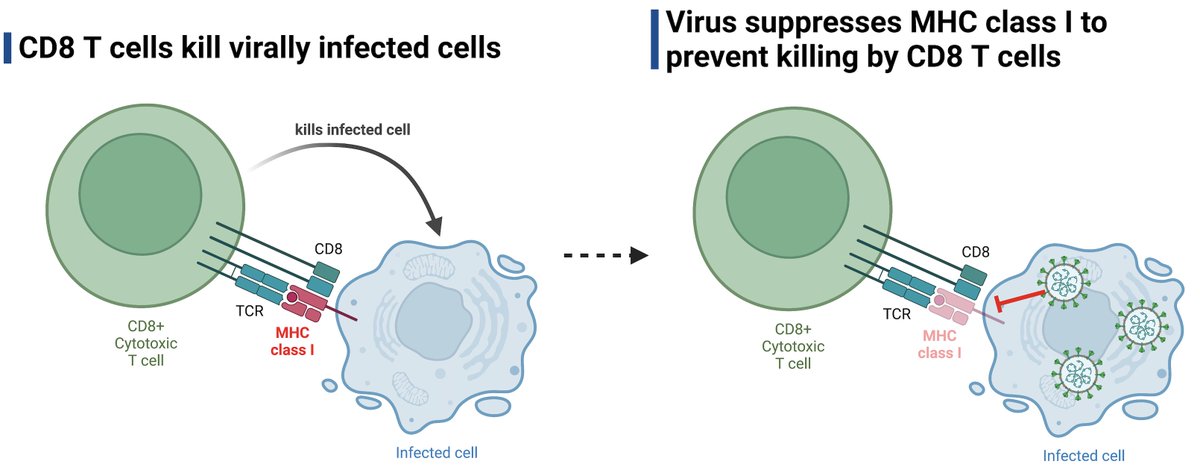
What could be the possible causes of PAIS? Similar to #longCOVID, we hypothesize a) pathogen reservoir/remnants, b) autoimmunity, c) dysbiosis of microbiome/virome, and d) unrepaired tissue damage. None are mutually exclusive. (6/) 

PAIS is an understudied and under-recognized spectrum of infectious diseases. We need more research on the causes, biomarkers and therapeutics to treat PAIS. #PAIS and #MECFS need to be taught at every medical school. We hope this review will help get that started 🙏🏼 (end)
• • •
Missing some Tweet in this thread? You can try to
force a refresh