🚨1/ New Study shows SARSCoV2 reprograms fat metabolism 🚨
#Covid_19 #metabolism
⬆️ Triglycerides
⬆️ PUFA ⬇️ Saturated fat
Blockade❌of fat synthesis blocks viral production
nature.com/articles/s4146…
#Covid_19 #metabolism
⬆️ Triglycerides
⬆️ PUFA ⬇️ Saturated fat
Blockade❌of fat synthesis blocks viral production
nature.com/articles/s4146…

2/ For a more detail...
Lipids & associated proteins have previously been identified as biomarkers of infection, including VLDL, HDL and various apolipoproteins, while both TAG and (serum) PUFA have been implicated as markers of severe disease outcomes
But what this paper adds
Lipids & associated proteins have previously been identified as biomarkers of infection, including VLDL, HDL and various apolipoproteins, while both TAG and (serum) PUFA have been implicated as markers of severe disease outcomes
But what this paper adds
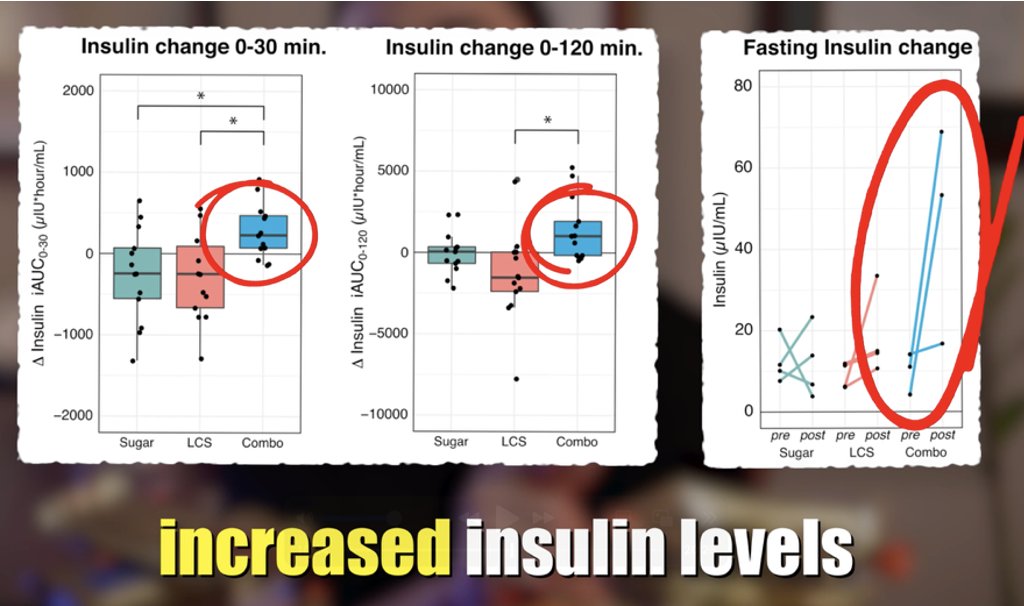
3/ Is an investigation (using mostly HEK293T-ACE2 and A549-ACE2 cells) of how the virus alters the lipidome and the importance of these changes in viral proliferation ... They found virus ⬆️TAGs, and PUFA chains were 2-8-fold more than saturated or monounsaturated species ... 

4/ Several of the genes encoded by the virus - orf6, nsp1, nsp5, nsp13, nsp5, orf9b, orfc - appeared particularly important in the TAG-PUFA changes. And more interestingly... 

5/ Drugs that alter fat metabolism, like an inhibitor of Fatty Acid Synthase (GSK2194069), strongly or completed blocked viral replication across viral strains. 

6/ Those are the data. Now my questions
👉 Wondering whether intake of industrial oils could predispose to more severe infection?
👉 Could diets that alter fat metabolism, by doing so, lower infection risk/severity?
👉Are docs going to start prescribing Orlistat for COVID?
👉 Wondering whether intake of industrial oils could predispose to more severe infection?
👉 Could diets that alter fat metabolism, by doing so, lower infection risk/severity?
👉Are docs going to start prescribing Orlistat for COVID?
• • •
Missing some Tweet in this thread? You can try to
force a refresh