Thrilled to share our work on DBS for #Alzheimer’s Disease
– likely the most important work in my career, so far.
Spearheaded by the one and only @sofiari91 w/ lots of collaborators, we sought out to find the optimal DBS stim site & network in AD
medrxiv.org/cgi/content/sh…
A 🧵
– likely the most important work in my career, so far.
Spearheaded by the one and only @sofiari91 w/ lots of collaborators, we sought out to find the optimal DBS stim site & network in AD
medrxiv.org/cgi/content/sh…
A 🧵
First, it is impossible to tag everyone that collaborated on this massive effort & it was a great honor to work on the data from the multicenter ADvance trial since ~2018 to make this possible. Big thanks to the Toronto team & Andres Lozano – and of course to all patients! 🙏 

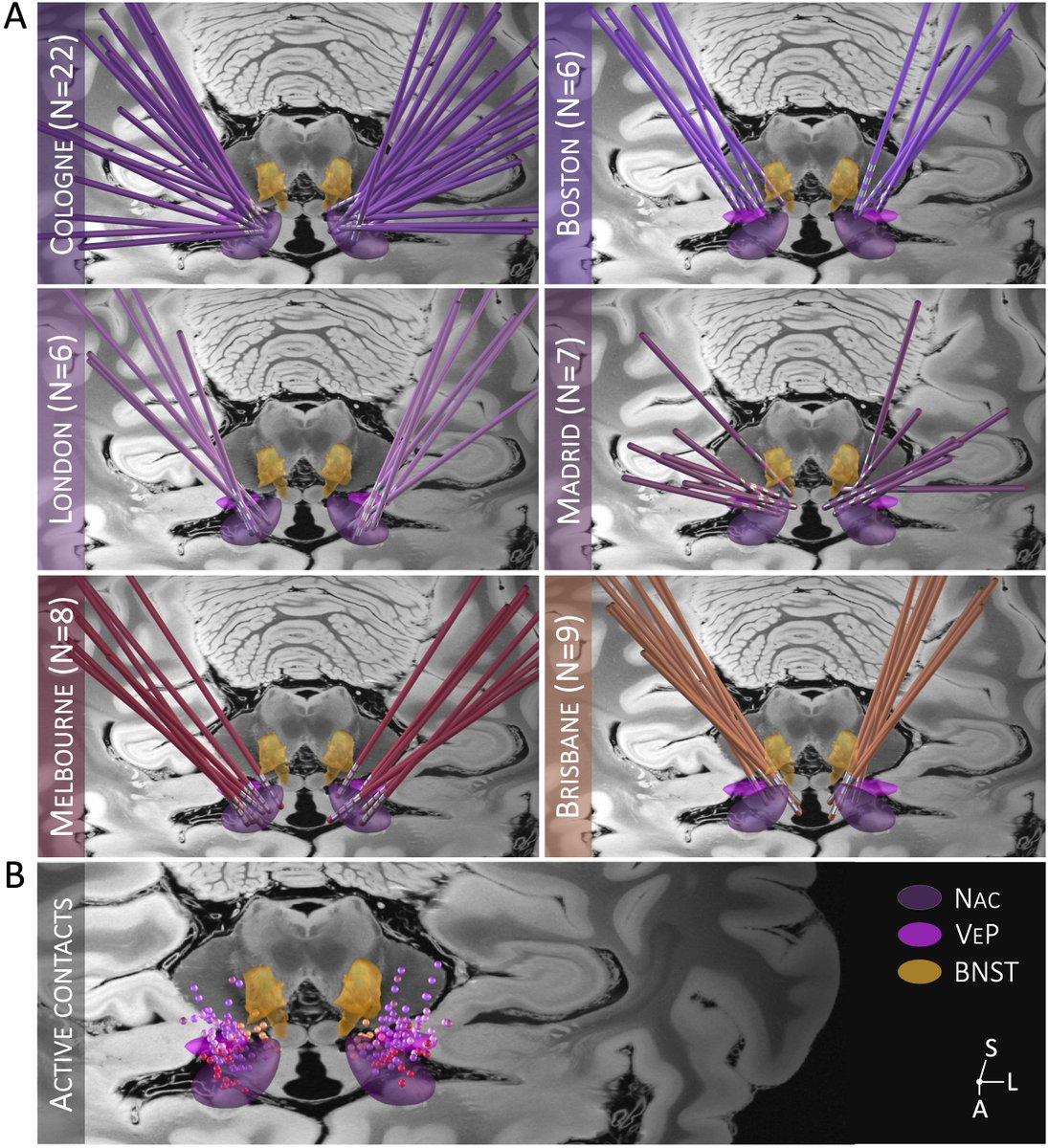
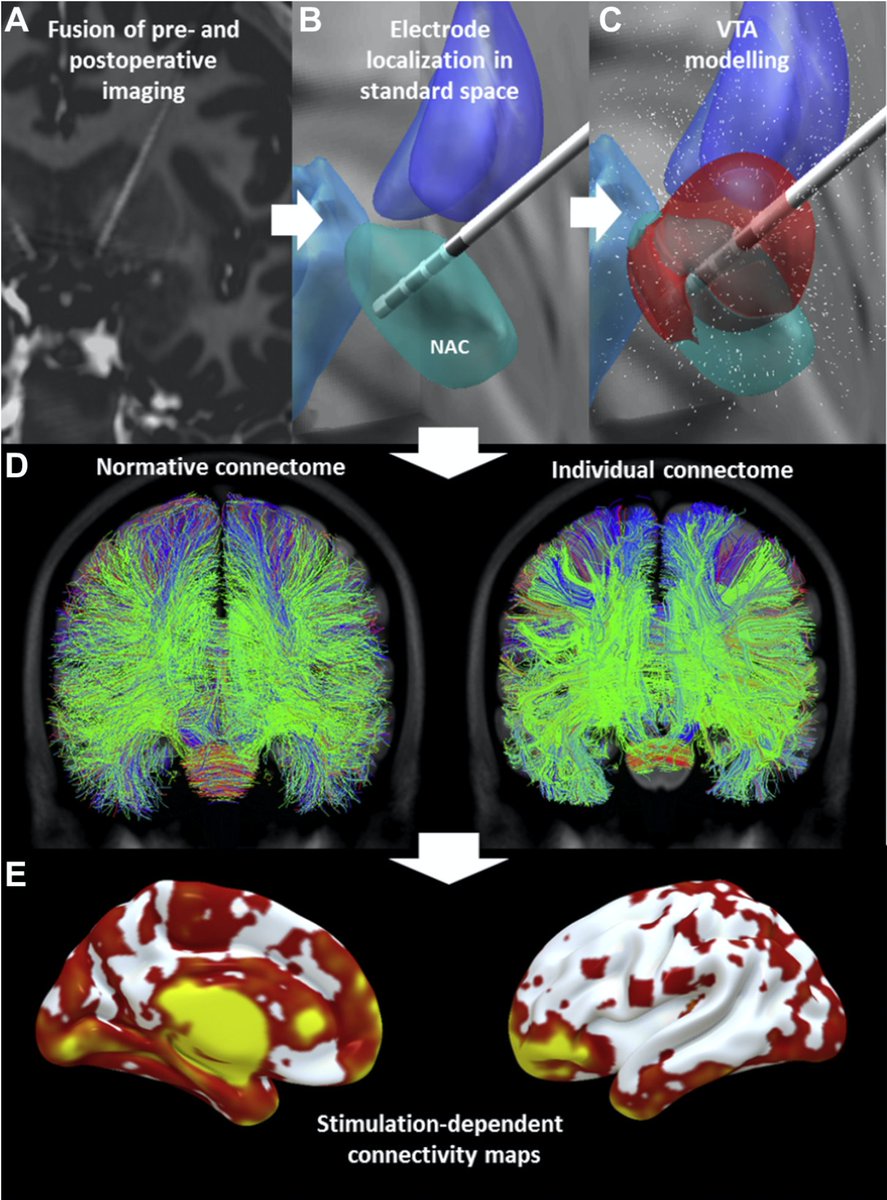
Based on electrode localizations made with @leaddbs we were able to register all stimulation sites into MNI space. Here, it was crucial to use @simonoxen’s WarpDrive method since registrations of atrophied brains is tough and millimeters matter. 
See this video on how we registered brains using WarpDrive – without this method, the present work would not have been possible (we know, since we had worked on the data before WarpDrive existed..)
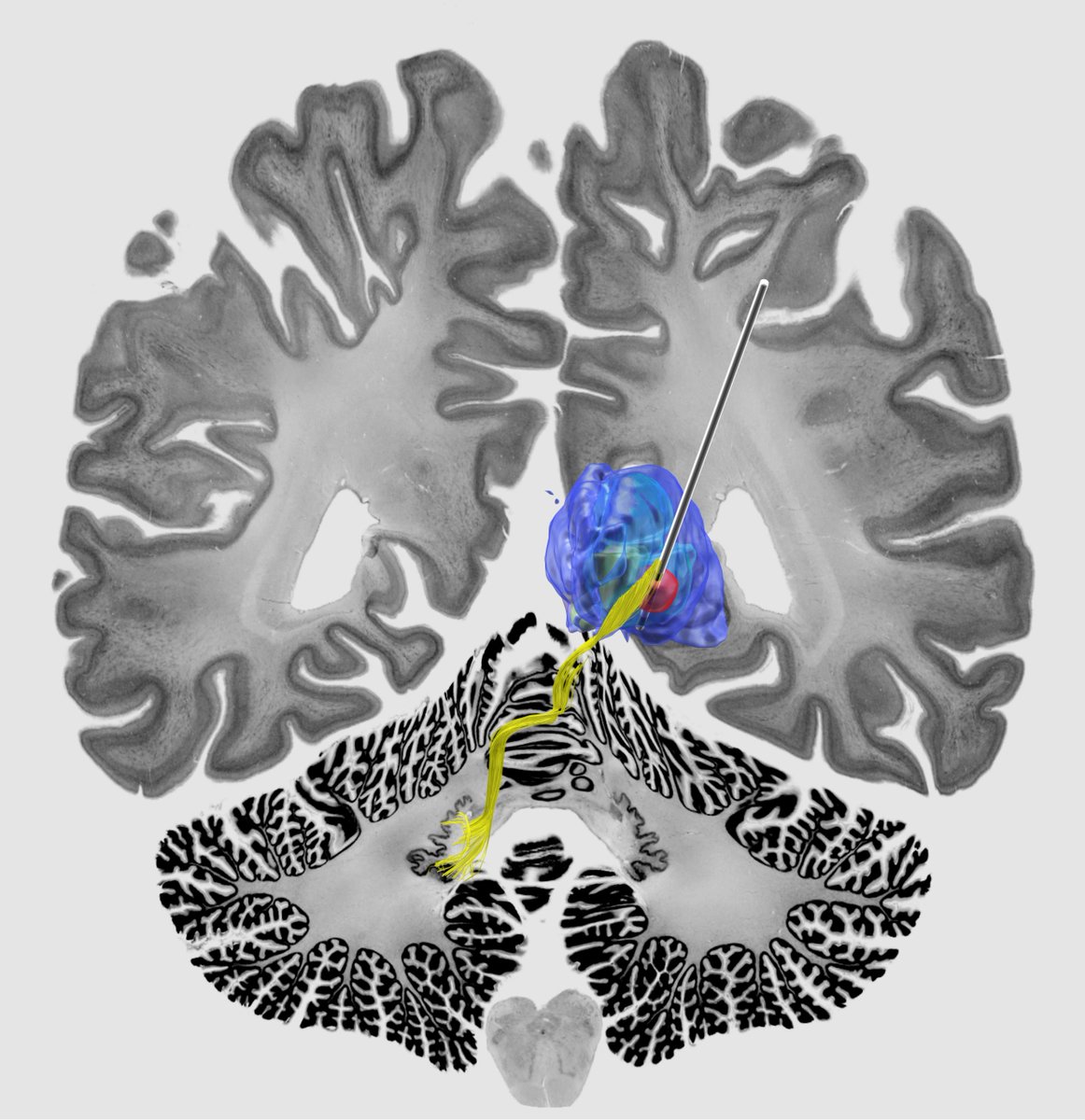
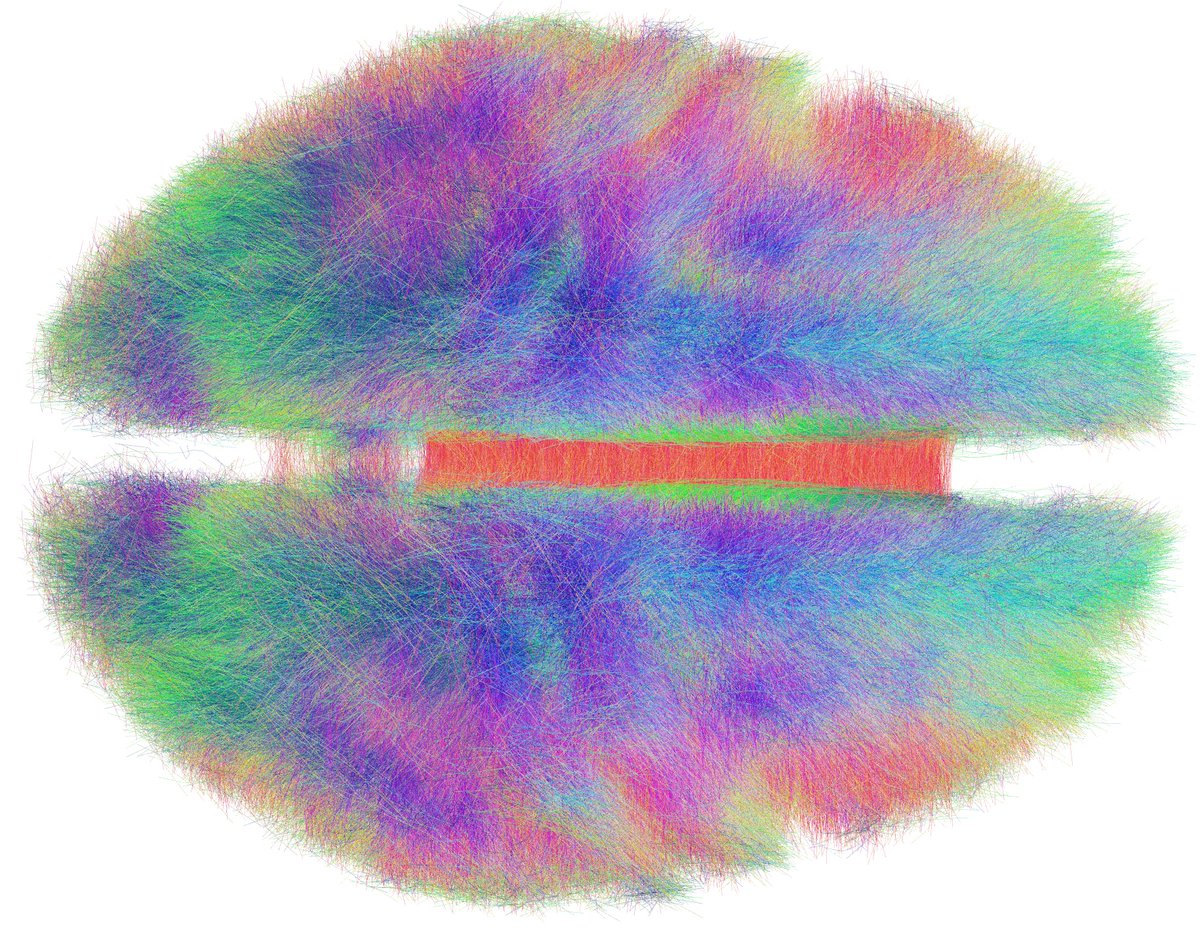
The second key ingredient was Kawin Setsompop’s gSlider connectome. Acquired by scanning for 18 hours at the @MGHMartinos center -> unprecedented detail and 760 um resolution. Now public (pubmed.ncbi.nlm.nih.gov/28261904/), Kawin graciously shared it w/ us for this project in 2019. 🙏 
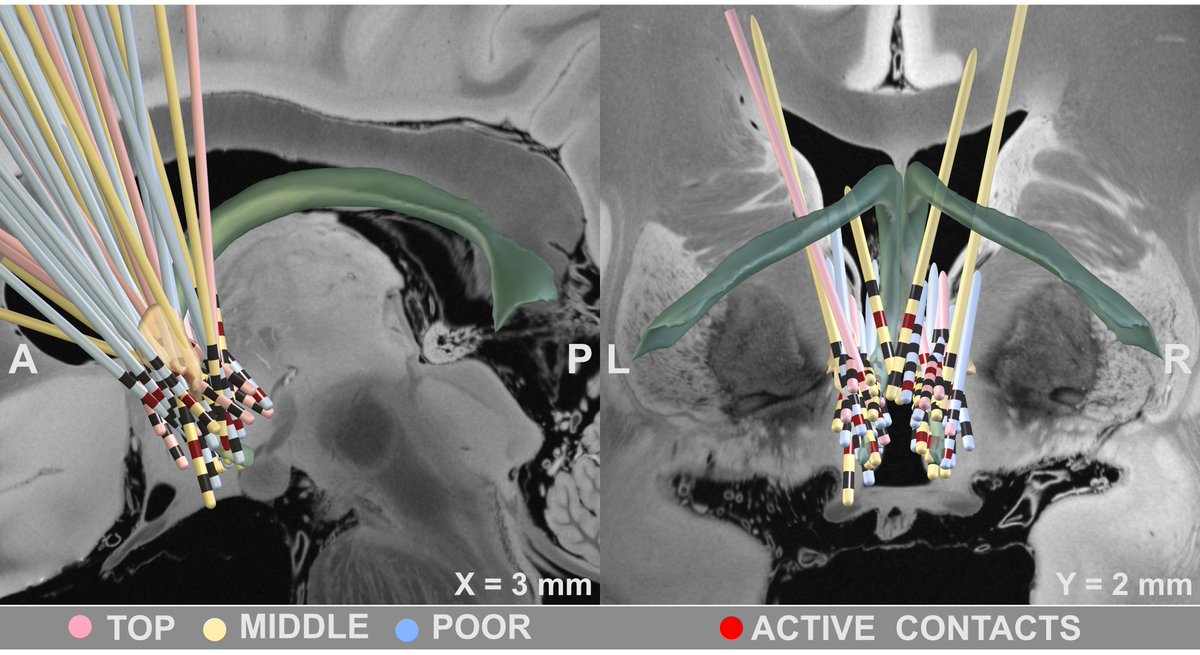
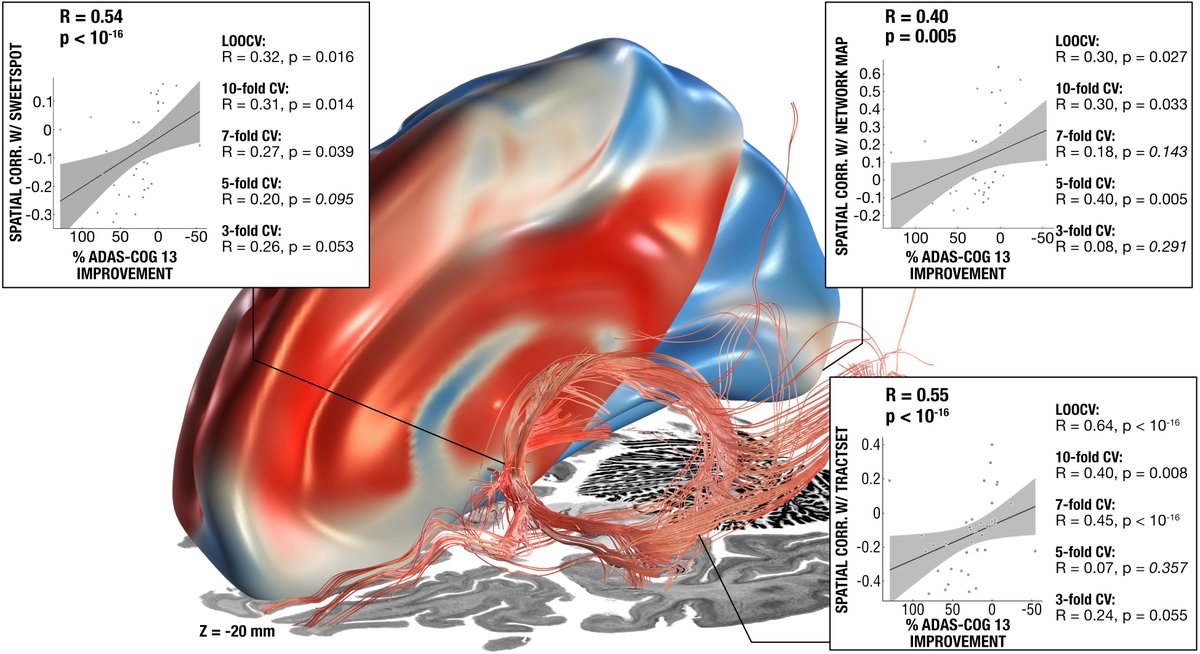
Set out with precisely registered electrodes & an ultra-high definition connectome, @sofiari91 set out to analyze the optimal sweet spot, tract set and fMRI network associated with optimal clinical response following DBS using @leaddbs. 
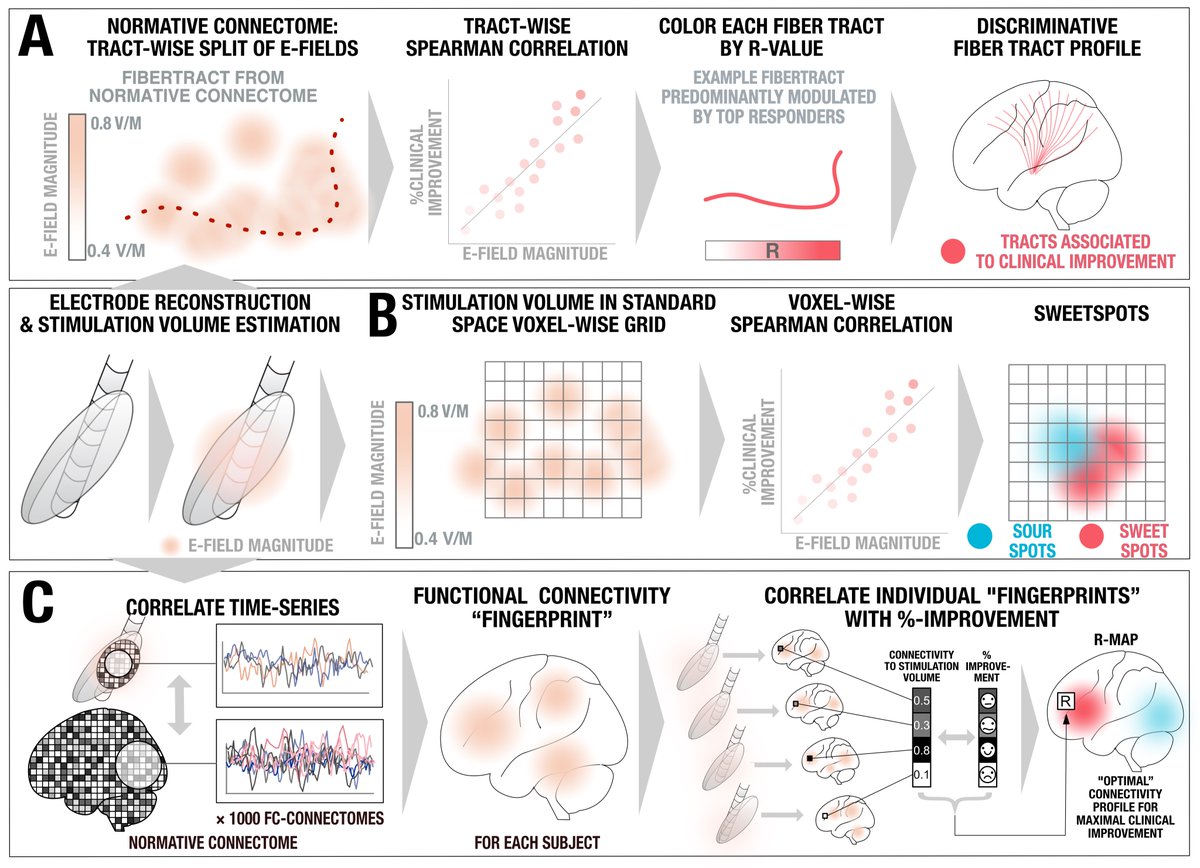
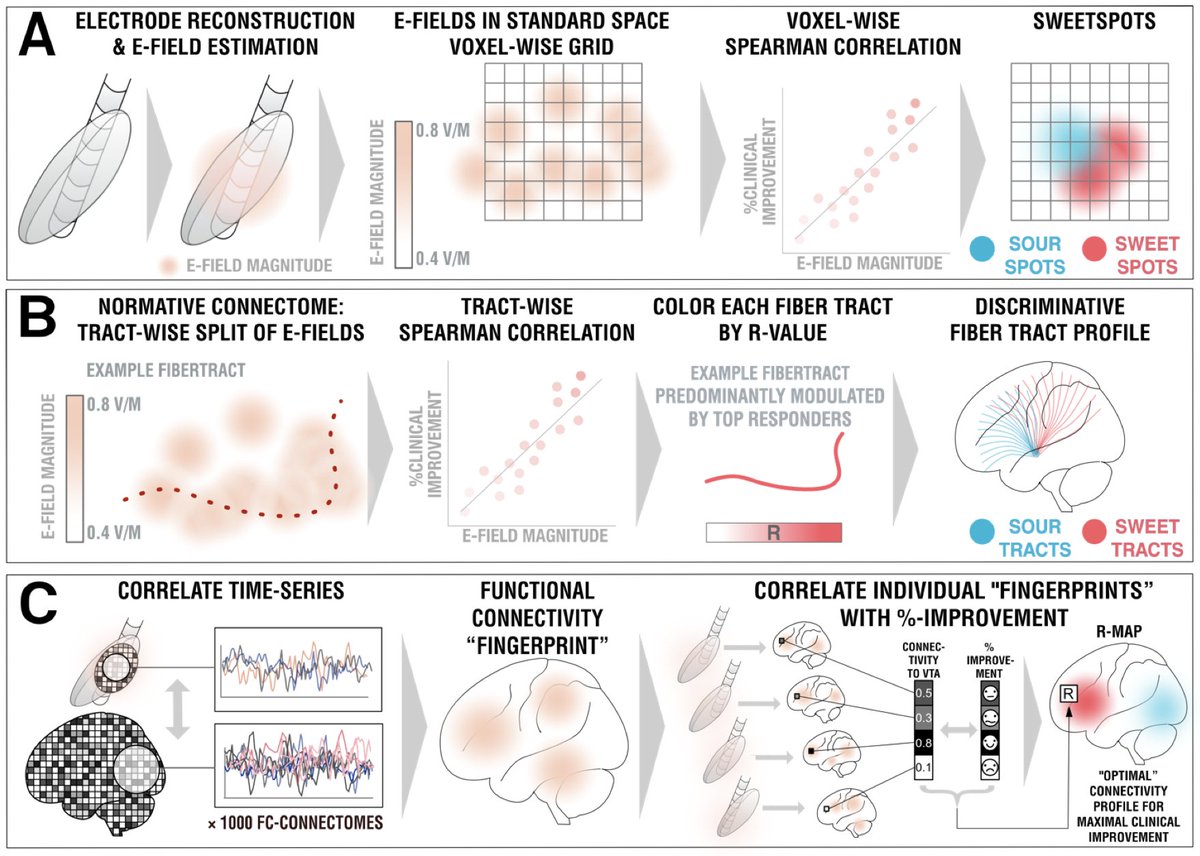
We split the uniquely large dataset into a balanced training and hold-out test cohort. Using DBS fiberfiltering, we isolated tracts associated with optimal outcomes in the training set and used this model to predict outcomes (in CV runs of the training set) & in the hold-out set. 

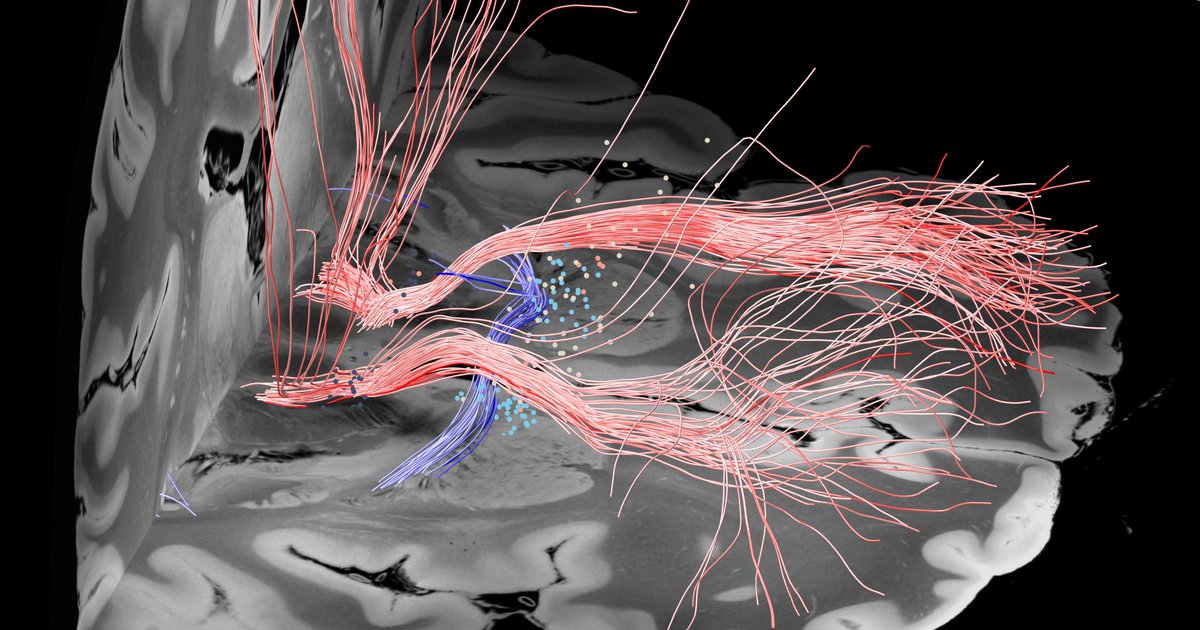
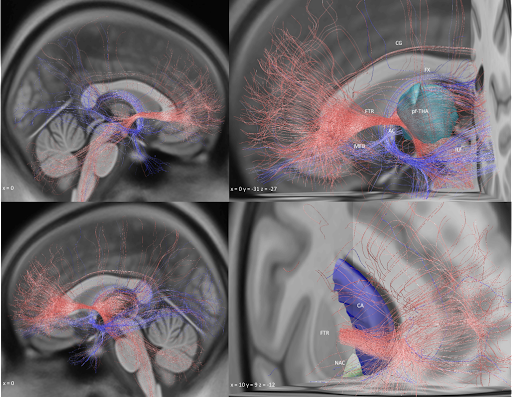
Key insight: Both forniceal system (Papez circuit) and stria terminalis seem key to modulate. This replicated well in the test-set after running predictions. Here the gSlider connectome could really shine – the stria terminalis, for instance, is not easy to reconstruct from dMRI. 
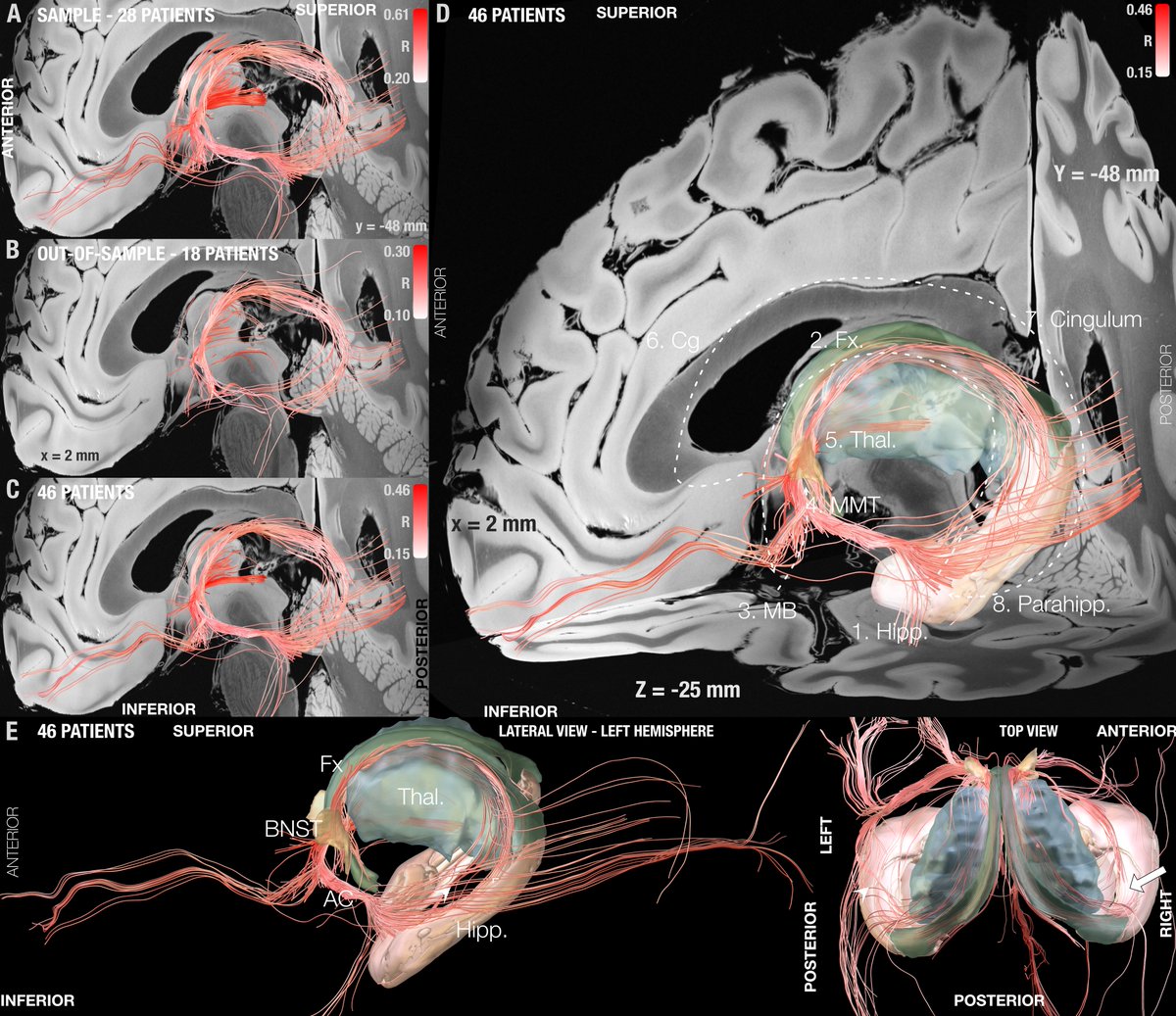
Important for surgeons beyond networks will be to know which coordinate or identifiable structure to target. So we added a sweetspot analysis, which further confirmed this intersection. 

In fact, the optimal site was situated directly at the crossing between bed nucleus and fornix, likely targeting two circuits relevant for memory. 

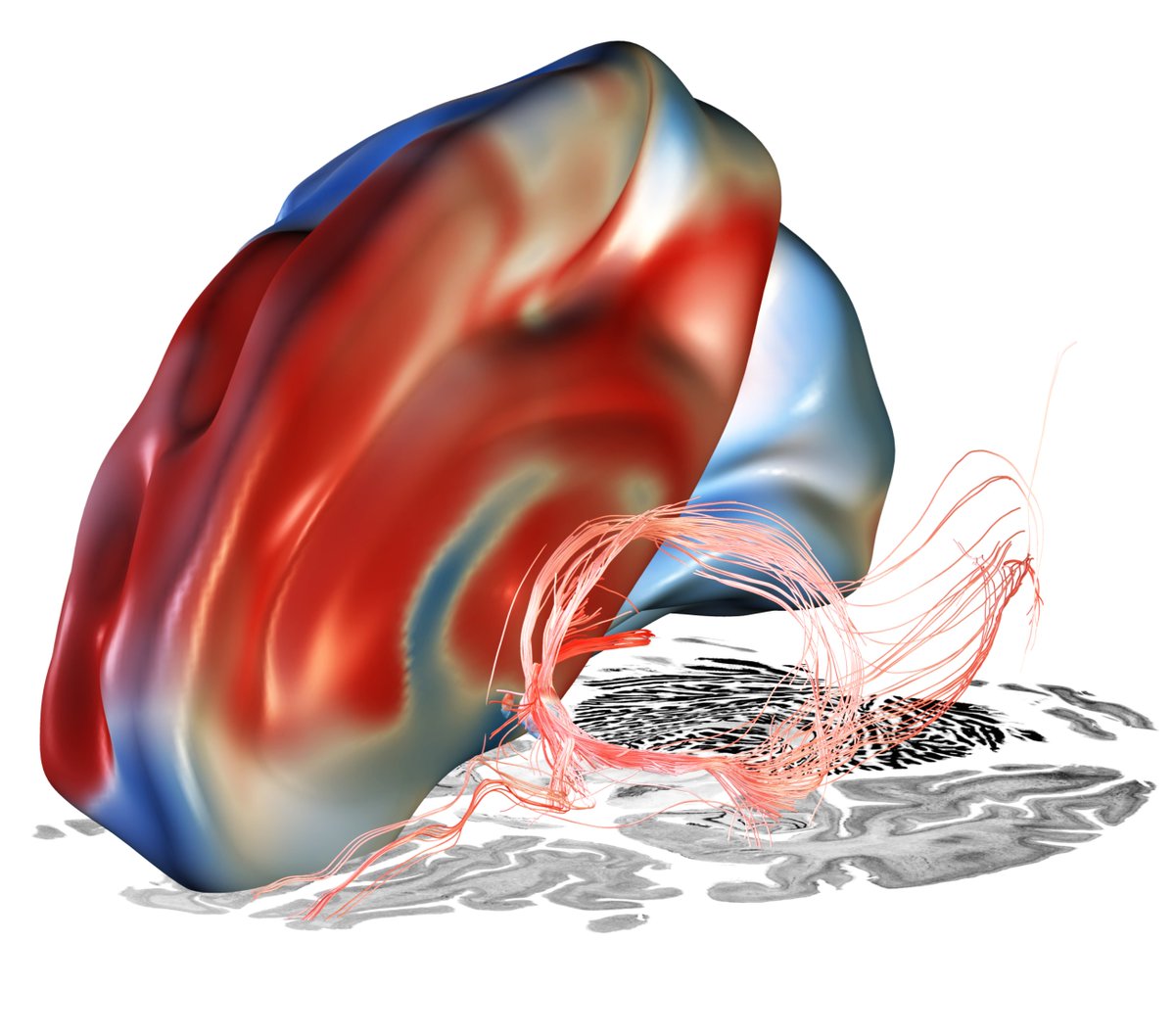
While tractography can show us monosynaptic connections, what about larger brain networks? We applied DBS network mapping and identified a specific network (compare robustness across test & retest), which, when modulated, was associated with optimal outcomes. 
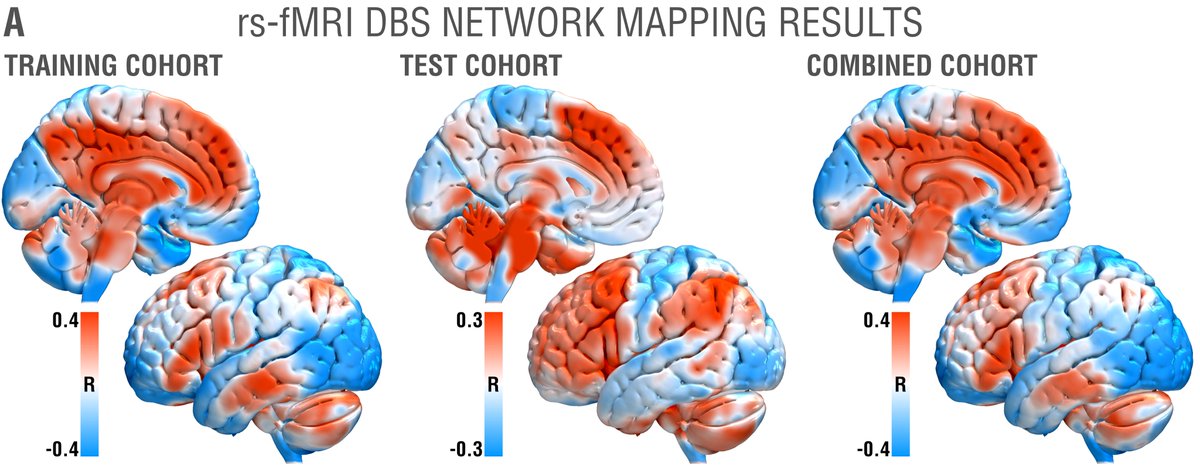
We ran this network through the @neurosynth decoder: Beyond unspecific anatomical terms, the first 8 functional terms (with one exception) were all related to memory & recall! This confirms specificity of the identified network to memory. 
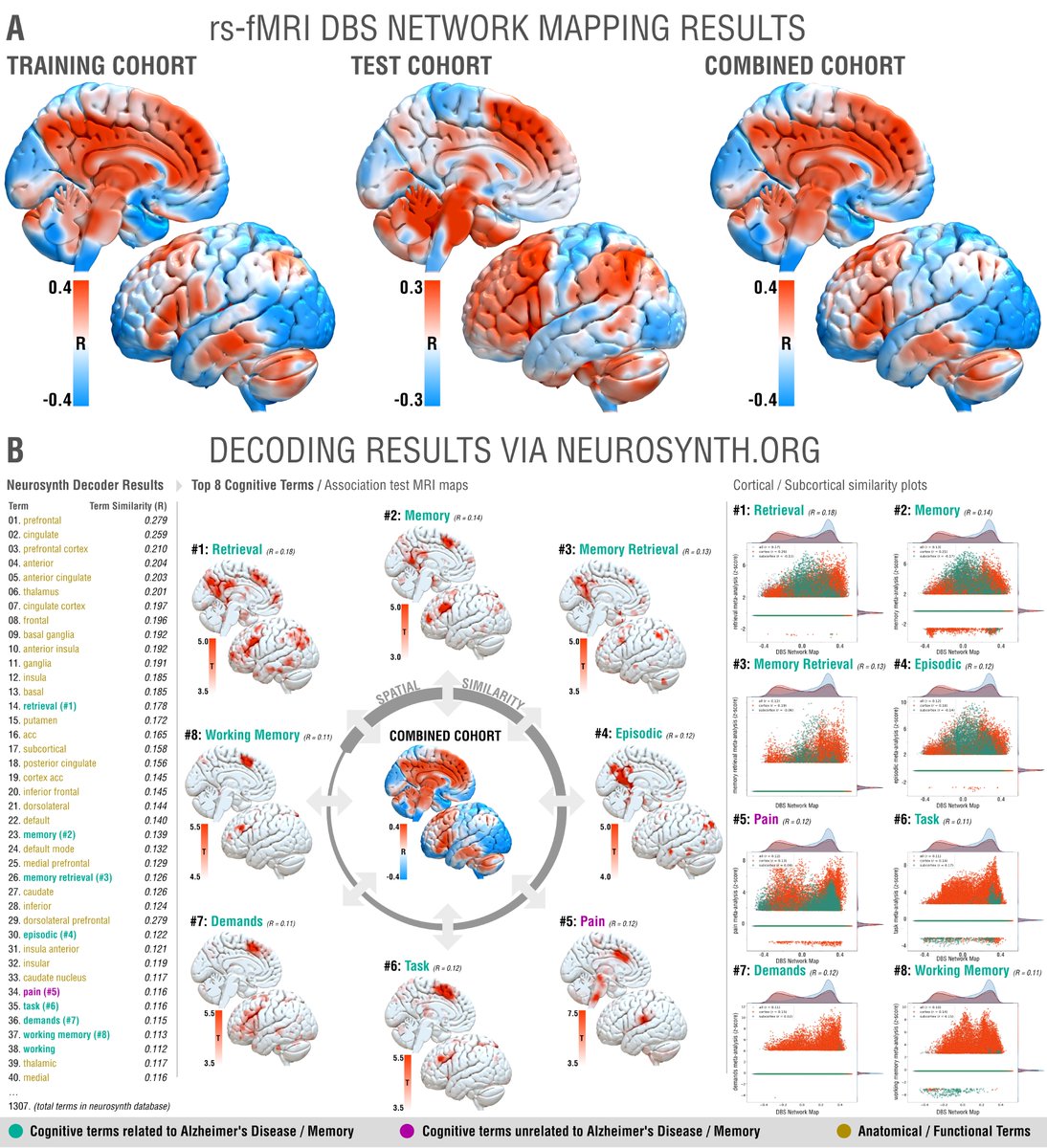
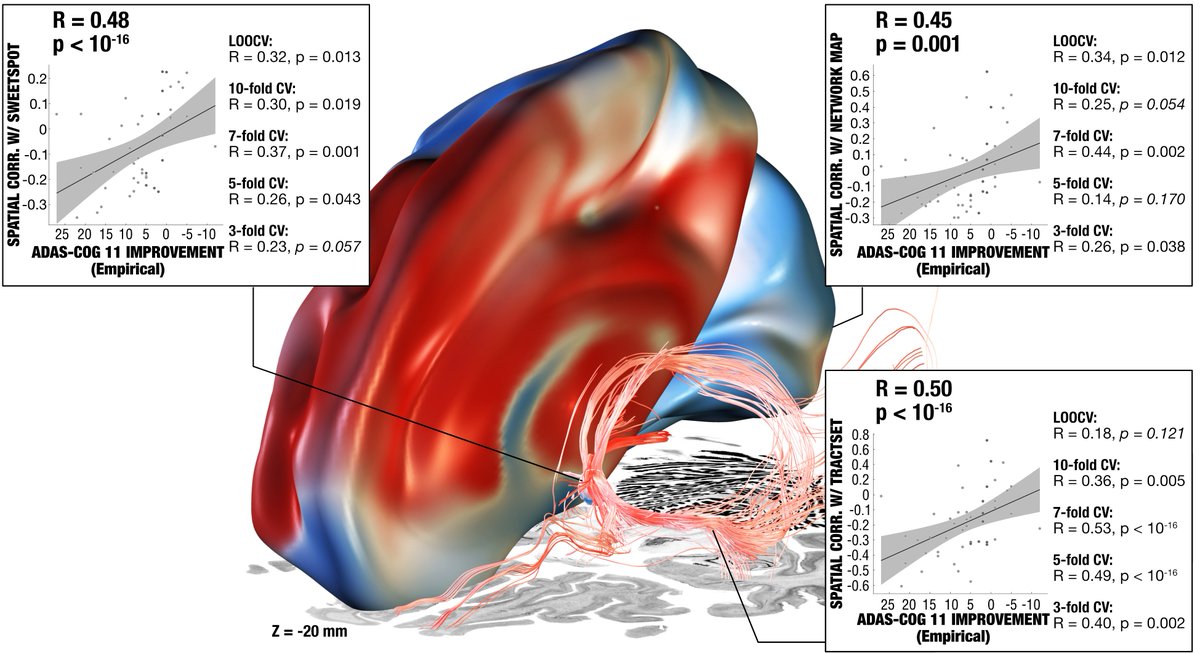
This summary figure shows two things. First, the functional network, tract & sweetspot, combined. But also, look at the many crossvalidations that were significant – we have not seen DBS network results as robust as this present finding, in my lab, before! 

Some of you may now wonder about flashbacks! The original beginning of Fornix-DBS started with an obesity case in which stimulation of the Forniceal region led to the occurence of flashbacks.
Also see great work by @Wissam1985 & @MichaelOkun on this @NEJM: pubmed.ncbi.nlm.nih.gov/31433930/
Also see great work by @Wissam1985 & @MichaelOkun on this @NEJM: pubmed.ncbi.nlm.nih.gov/31433930/

Rerunning fiberfiltering on the flashback data revealed that it is the posterior limb of the internal capsule – not the fornix – that elicits these flashbacks. Finding super robust & significant. 
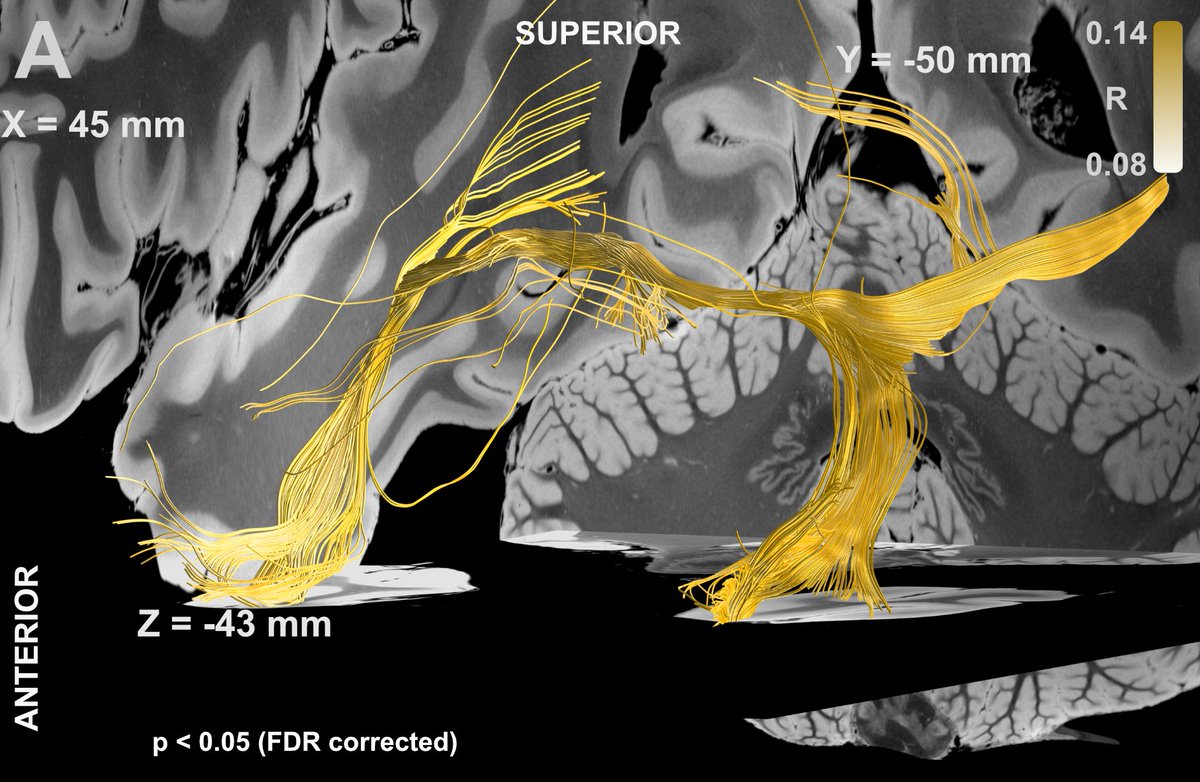
That came as a surprise! What does the AC do, again, you may ask? Well, it connects the bilateral temporal poles & lobes! 
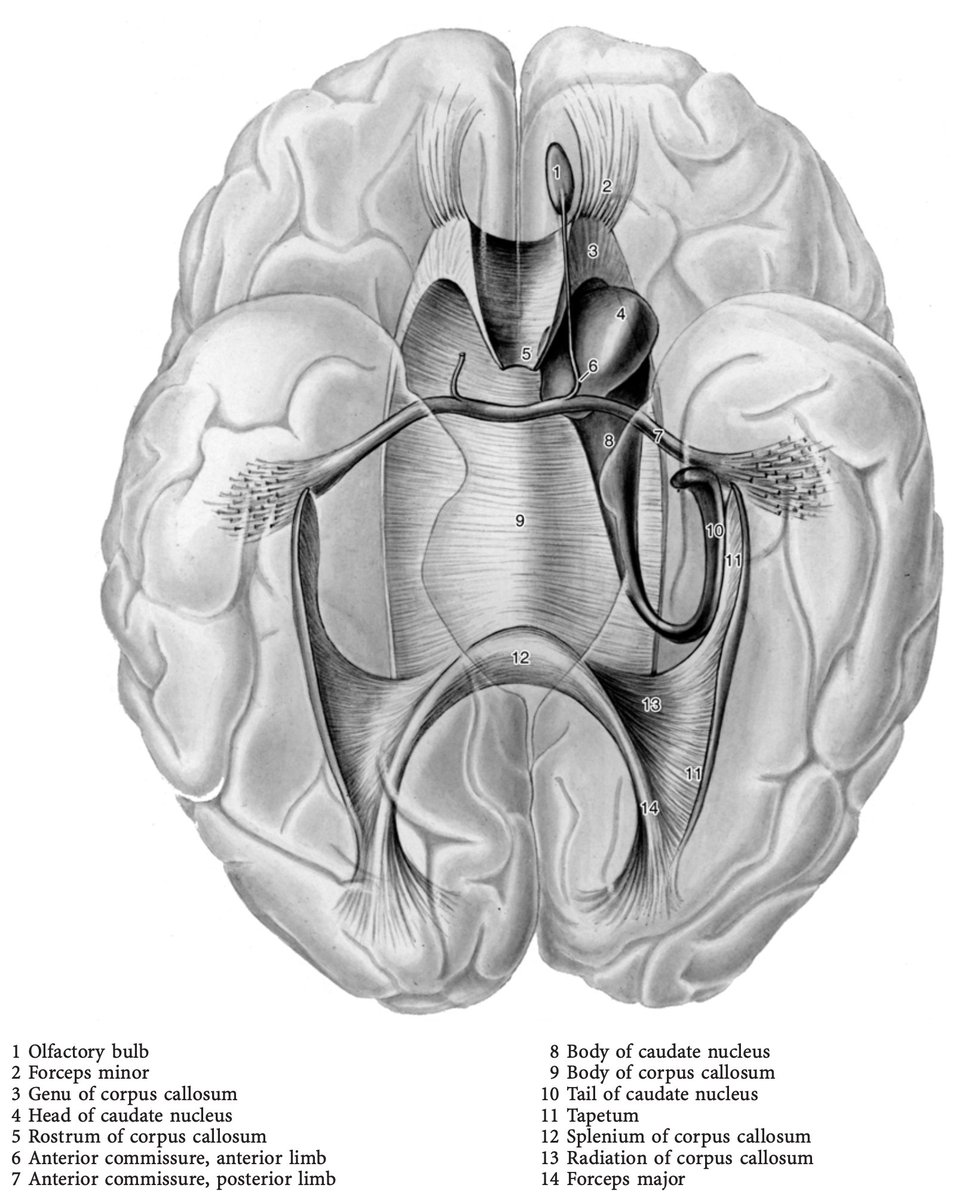
Thinking about this, we realized that Wilder Penfield’s work reported many flashback events specifically when modulating these parts in the temporal lobes (as published in @Brain1878 1963: academic.oup.com/brain/article-…)! 
So in conclusion, while the Fornix (and Stria) seem important for clinical improvement, the flashback phenomena may not have much to do with it. Clinical serendipity at it’s finest! (ping @mar1hz) 

I would like to highlight massive methods support by twitterless Konstantin Butenko, @NingfeiL, @NaN_Value, @simonoxen & @c_neudorfer which worked countless hours on showing robustness of our cross-validations and predictions across datasets. 



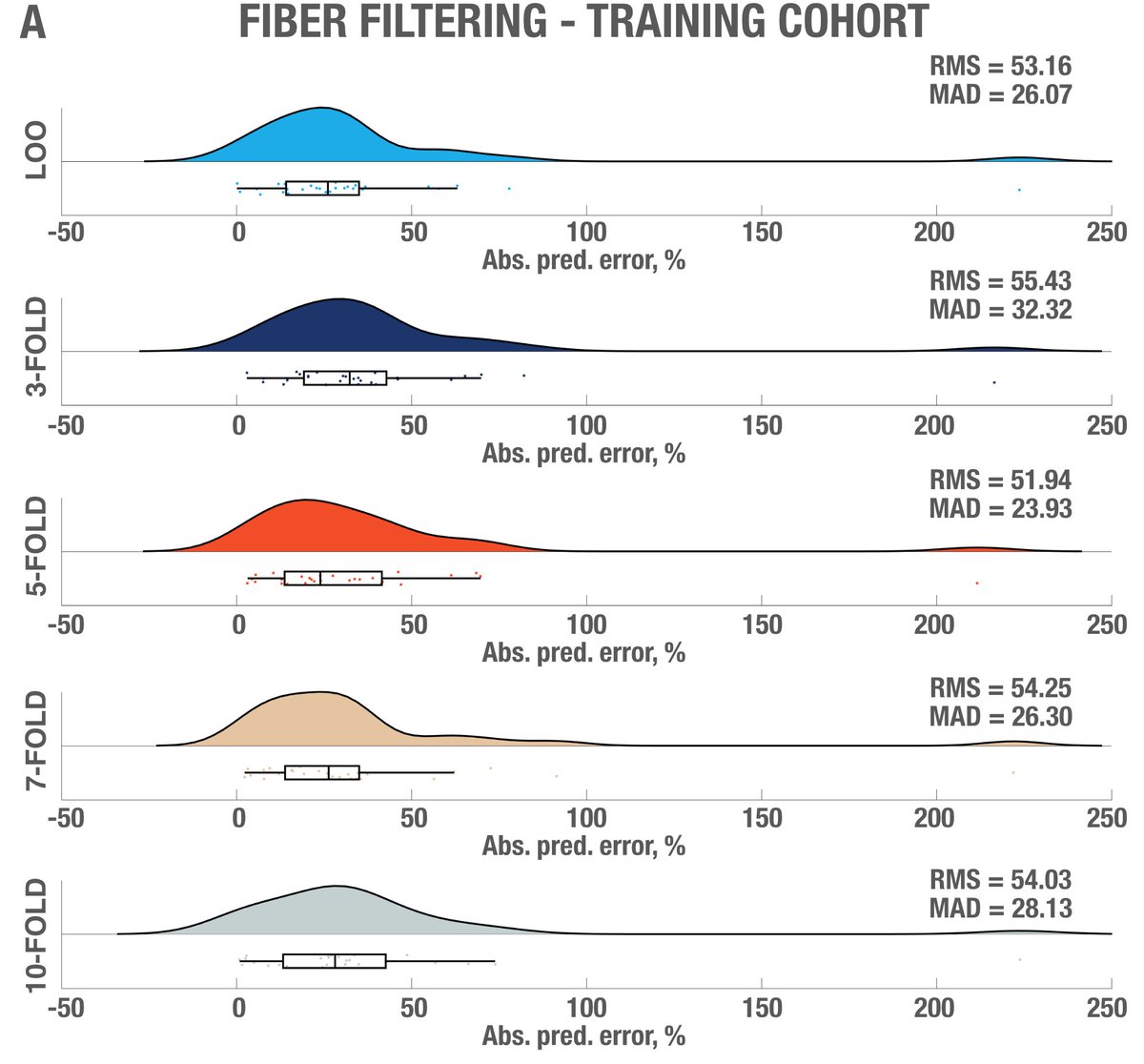

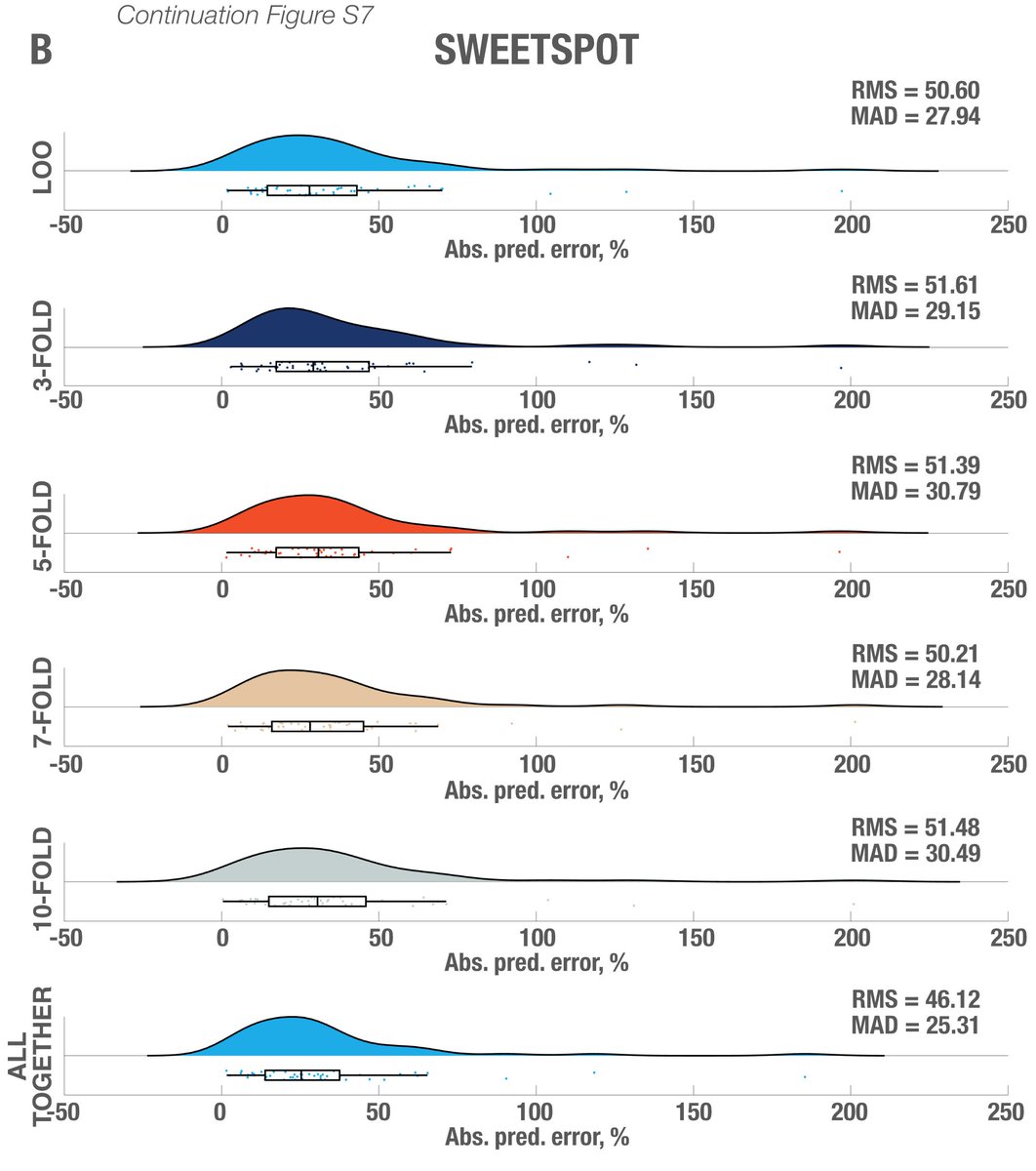

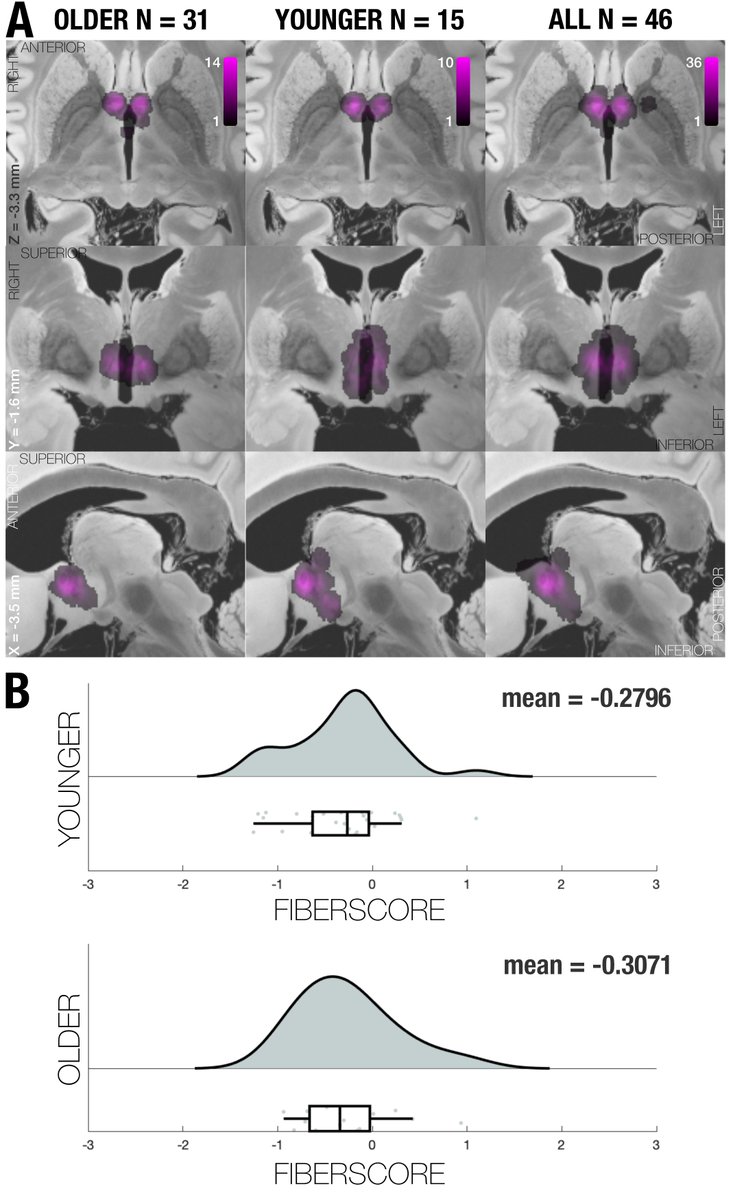
While this was requested by reviewers, it may have been the most rigorous “prediction” analysis carried out in the DBS field, so far. Subanalyses replicated results when using absolute vs. relative scores; and no clear effects of age. 


Finally, I would like to highlight once more the tremendous effort @sofiari91 put into this! Ana joined the @netstim_org as a master’s student (!) a few years back and this is her first paper.
I can’t recount how many hours she worked on this during many revisions.. ✨⚡️☄️💥
I can’t recount how many hours she worked on this during many revisions.. ✨⚡️☄️💥

The end – Thank you so much for your attention, feedback and retweets!
• • •
Missing some Tweet in this thread? You can try to
force a refresh