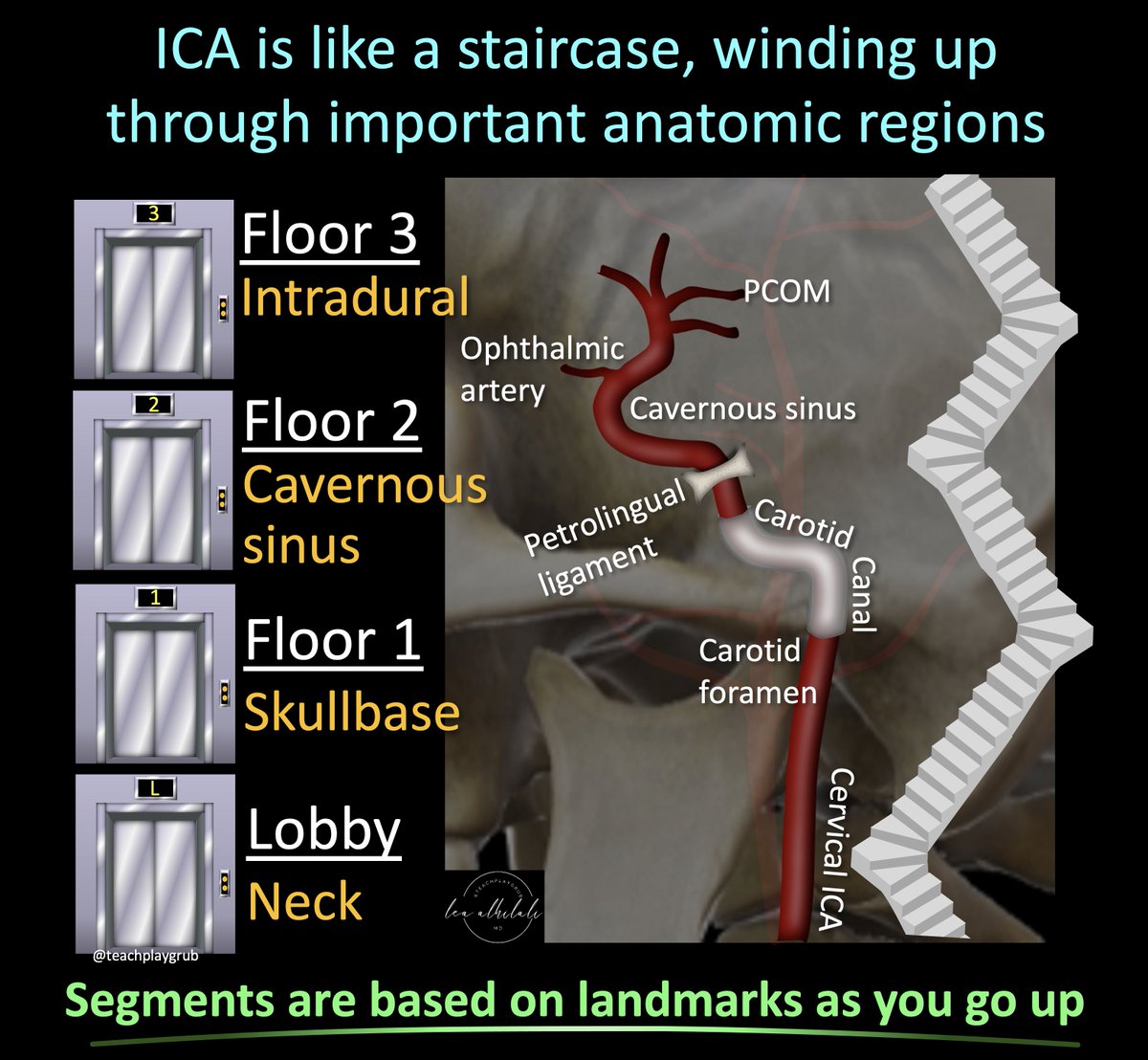
1/Asking “How old are you” can be dicey—both in real life & on MRI! Do you know how to tell the age of blood on MRI?
Here’s a #tweetorial on how to date blood on MRI
#medtwitter #neurorad #radtwitter #RSNA2022 #RSNA22 #radres #neurosurgery #neurology #meded #neurotwitter #FOAMed
Here’s a #tweetorial on how to date blood on MRI
#medtwitter #neurorad #radtwitter #RSNA2022 #RSNA22 #radres #neurosurgery #neurology #meded #neurotwitter #FOAMed
2/If you ask someone how to date blood on MRI, they’ll spit out a crazy mnemonic about babies that tells you what signal blood should be on T1 & T2 imaging by age
But mnemonics are crutch—they help you memorize, but not understand. If you understand, you don’t need to memorize
But mnemonics are crutch—they help you memorize, but not understand. If you understand, you don’t need to memorize
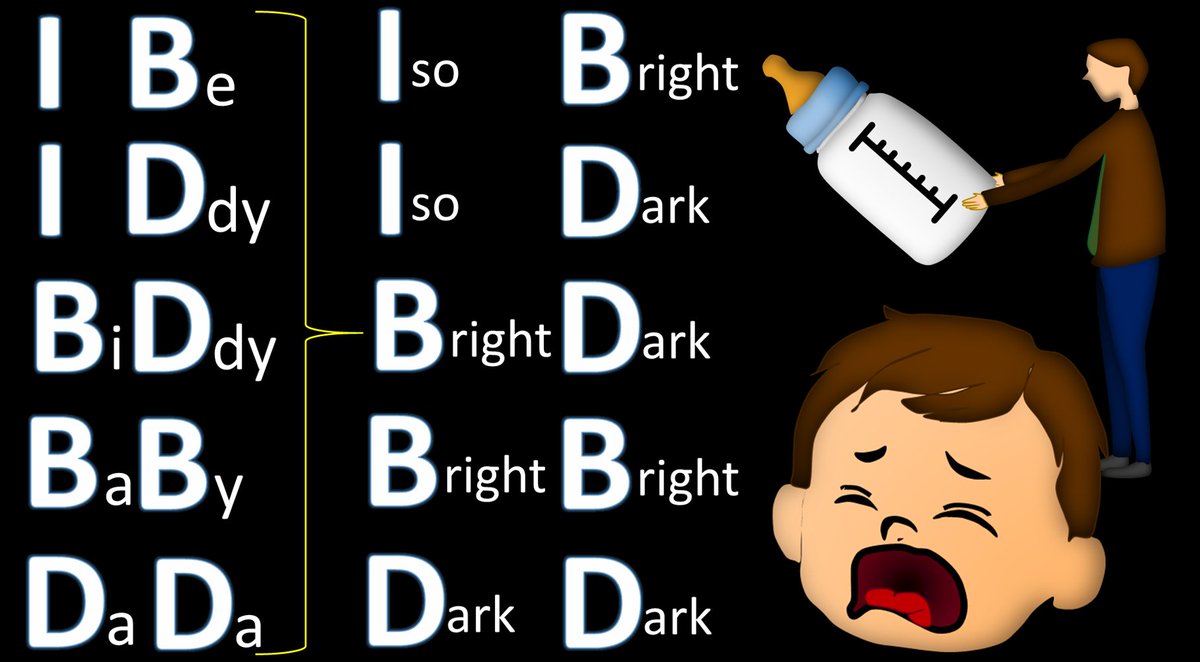
3/If you look at the mnemonic, you will notice one thing—the T1 signal is all you need to tell if blood is acute, subacute or chronic
T2 signal will tell if it is early or late in each of those time periods—but that type of detail isn’t needed in real life. So let’s look at T1
T2 signal will tell if it is early or late in each of those time periods—but that type of detail isn’t needed in real life. So let’s look at T1

4/To understand how blood looks on MRI at different ages, you need 2 basic MRI rules.
Remember T1 loves protein. More protein = brighter on T1. I remember this bc T1 looks like the T & I in proTeIn.
T2 loves water. Fluid is bright on T2. This is easy bc there’s a 2 in H2O.
Remember T1 loves protein. More protein = brighter on T1. I remember this bc T1 looks like the T & I in proTeIn.
T2 loves water. Fluid is bright on T2. This is easy bc there’s a 2 in H2O.

5/Acute blood is in the first hours. It is basically blood that has just poured out of the artery.
If you think about how acute bleeding looks in real life, you know the properties of acute blood—it’s basically water w/a little protein to gives it the red color & thickness
If you think about how acute bleeding looks in real life, you know the properties of acute blood—it’s basically water w/a little protein to gives it the red color & thickness

6/How does T1 feel about acute blood?
Well, acute blood is a lot of water w/a little protein. So you will get some love from T1 for the litte protein. But it won’t be super bright bc the protein content isn’t that high—it’s diluted.
So it acute blood is isointense on T1.
Well, acute blood is a lot of water w/a little protein. So you will get some love from T1 for the litte protein. But it won’t be super bright bc the protein content isn’t that high—it’s diluted.
So it acute blood is isointense on T1.

7/Here’s an example of acute blood on T1.
The hematoma is very dense on CT, consistent w/acute timing.
On T1, its isointense to brain. It’s not bright bc protein content is relatively low. But it isn’t dark either, bc proteins are in blood that will give it some signal
The hematoma is very dense on CT, consistent w/acute timing.
On T1, its isointense to brain. It’s not bright bc protein content is relatively low. But it isn’t dark either, bc proteins are in blood that will give it some signal

8/After a few days, you get subacute blood. In the subacute period, blood gets oxidized. It’s like what happens to an apple when you leave it out, or why a steak turns dark when it’s left out. Subacute blood is oxidized blood that has been left out for a few days like a steak. 

9/When blood gets oxidized in the subacute period, hemoglobin becomes methemoglobin. This change in hemoglobin marks the transition from acute to subacute blood. 

10/Subacute period is like aging a steak. Cells will begin to lyse & water content will be lost. This is exactly what happens w/a steak. It’s why we age a steak—the broken down proteins & lower water content lead to a more tender & flavorful steak 

11/Both of these processes—letting proteins out of cells & decreasing water content—will increase the protein density.
More protein means higher T1 signal.
This contributes to giving subacute hematomas a very bright signal on T1.
More protein means higher T1 signal.
This contributes to giving subacute hematomas a very bright signal on T1.

12/Although more protein from the aging process leads to high T1, high T1 comes also from new electrons from the oxidation to methemoglobin.
I just remember that using Meth is basically a way to age humans like dry aging a steak
So Meth(emoglobin) will lead to increased T1.
I just remember that using Meth is basically a way to age humans like dry aging a steak
So Meth(emoglobin) will lead to increased T1.

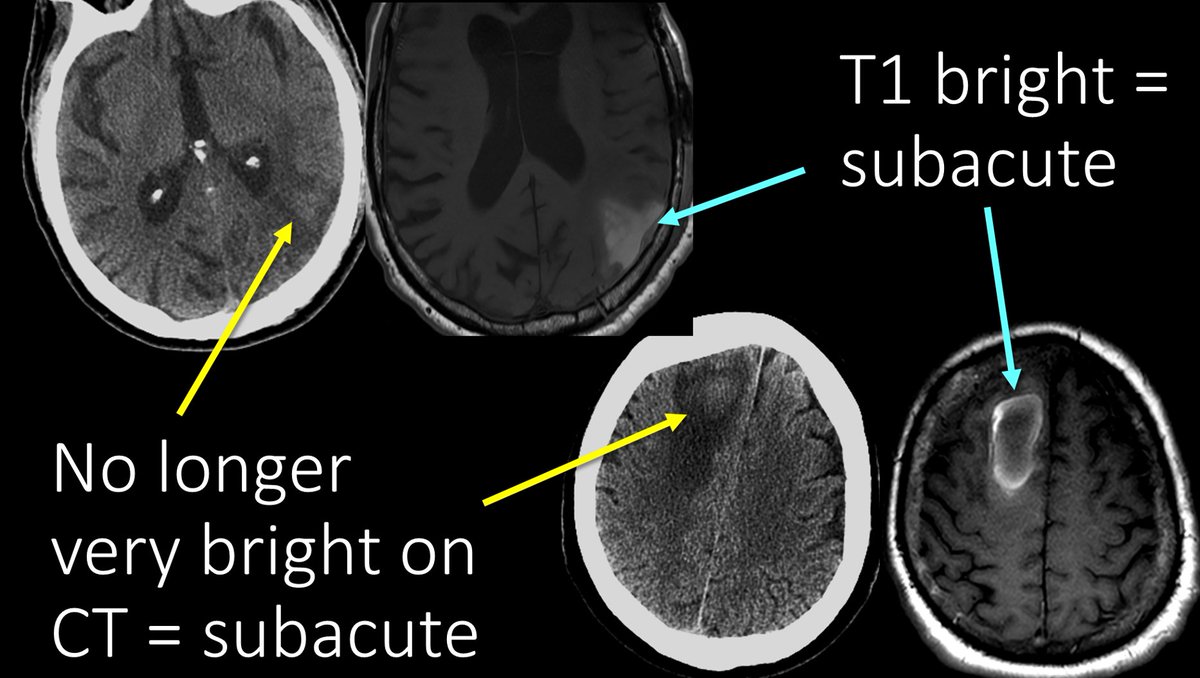
13/Here are examples of subacute blood on MRI.
You can see these hematomas are only subtly bright on CT now, as their acute clots have begun to be broken down.
On MRI, these have increased T1 signal related to the increased protein and increased Meth.
You can see these hematomas are only subtly bright on CT now, as their acute clots have begun to be broken down.
On MRI, these have increased T1 signal related to the increased protein and increased Meth.
14/Chronic blood is after a few weeks. In the subacute phase, cells lyse. In the chronic phase, the proteins themselves lyse, including heme. This releases the iron from the heme.
The iron molecules from the broken heme start to clump together to make ferritin or hemosiderin.
The iron molecules from the broken heme start to clump together to make ferritin or hemosiderin.

15/Neither T1 or T2 sequences like metal like ferritin/hemosiderin.
You can remember this bc metal doesn’t mix well with protein (metal cuts right through protein) or water (together water and iron make rust).
You can remember this bc metal doesn’t mix well with protein (metal cuts right through protein) or water (together water and iron make rust).

16/Bc neither T1 or T2 like ferritin/hemosiderin, you will end up getting a dark signal on both sequences in the chronic phase.
In fact, no sequence really likes hemosiderin, and it will be dark on all sequences.
In fact, no sequence really likes hemosiderin, and it will be dark on all sequences.

17/Here’s an example of a chronic hematoma.
Six years ago, it looked like an acute hematoma—isointense on T1 (Even though there is a lot of fluid in the acute blood, there are also some proteins to give some signal).
Now it is dark on T1, bc everyone hate hemosiderin.
Six years ago, it looked like an acute hematoma—isointense on T1 (Even though there is a lot of fluid in the acute blood, there are also some proteins to give some signal).
Now it is dark on T1, bc everyone hate hemosiderin.

18/So remember: acute is a few hours, subacute is a few days, & chronic is a few weeks.
Acute blood is like flowing blood but outside the vessel.
Subacute blood has started oxidation & cell lysis
Chronic blood has broken down everything so that even iron is out on its own
Acute blood is like flowing blood but outside the vessel.
Subacute blood has started oxidation & cell lysis
Chronic blood has broken down everything so that even iron is out on its own

19/Knowing what acute, subacute, and chronic blood consist of can help you to remember your T1 signal:
Acute is fluid w/little protein = isointense
Subacute has lots of protein from cell lysis & water loss & methemoglobin = bright
Chronic is filled w/iron no one likes = dark
Acute is fluid w/little protein = isointense
Subacute has lots of protein from cell lysis & water loss & methemoglobin = bright
Chronic is filled w/iron no one likes = dark

20/T1 feels about blood like you feel about a good steak.
Acute is raw meat—has potential, but you won’t eat it yet = isointense.
Subacute has freed all the proteins for good taste, you want to dig in = bright.
Chronic has broken down too much & is rotten—no thanks = dark.
Acute is raw meat—has potential, but you won’t eat it yet = isointense.
Subacute has freed all the proteins for good taste, you want to dig in = bright.
Chronic has broken down too much & is rotten—no thanks = dark.

21/Of course, there are subtleties to this related to oxygen tension, blood flow to the region, hematocrit, etc. But as a rule of thumb, think of blood on T1 MRI like you would a good steak—bon apetit!
• • •
Missing some Tweet in this thread? You can try to
force a refresh