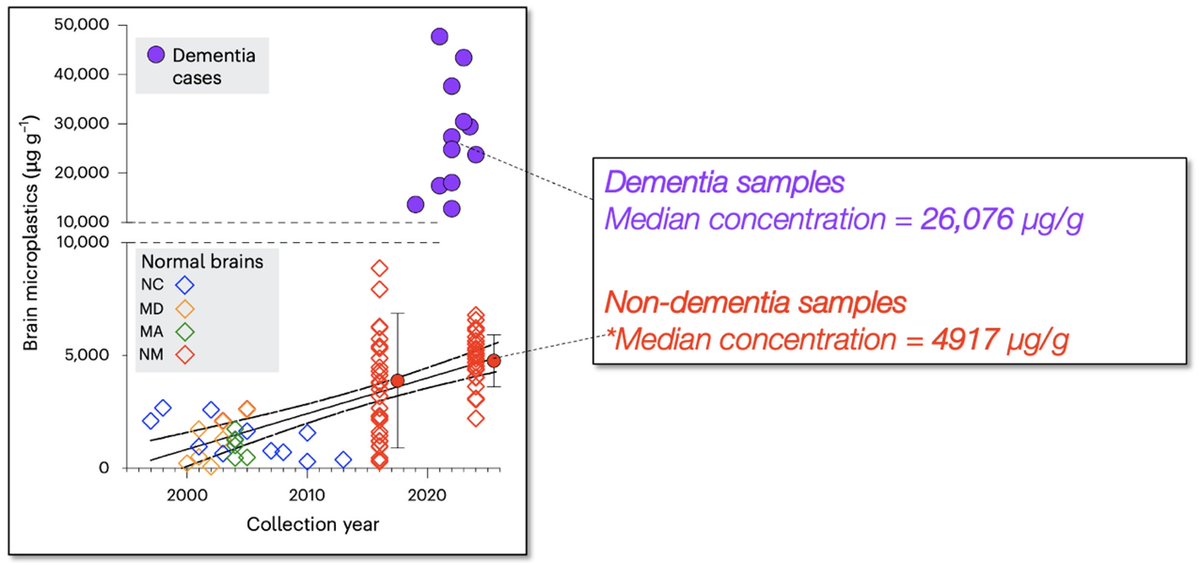
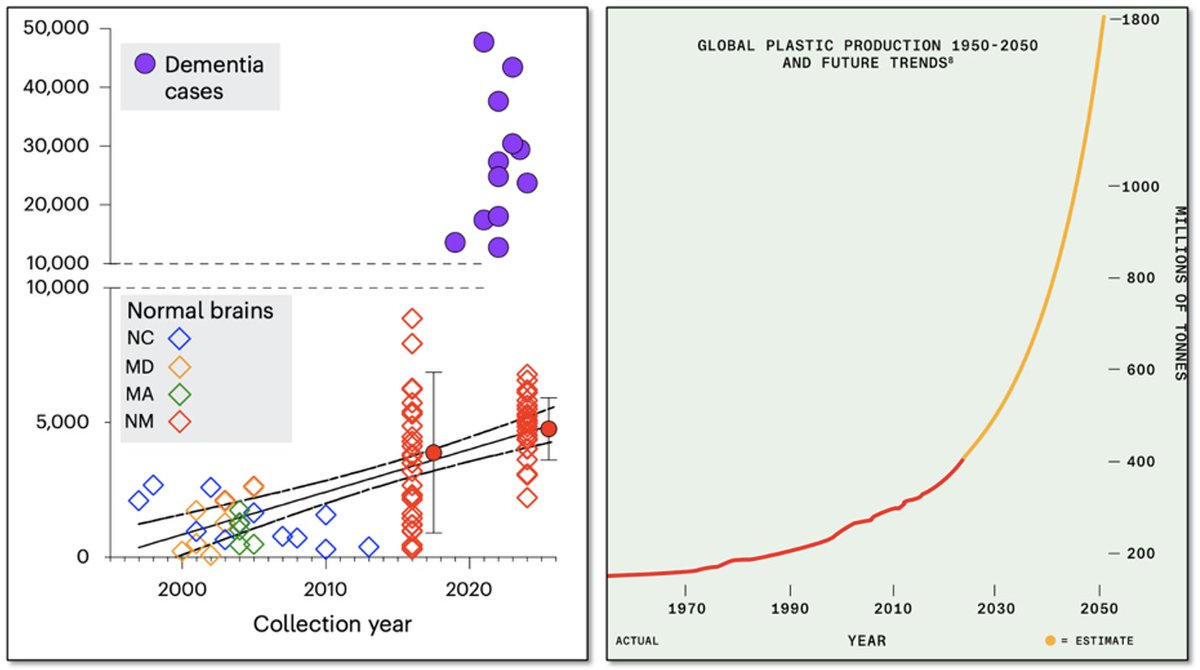
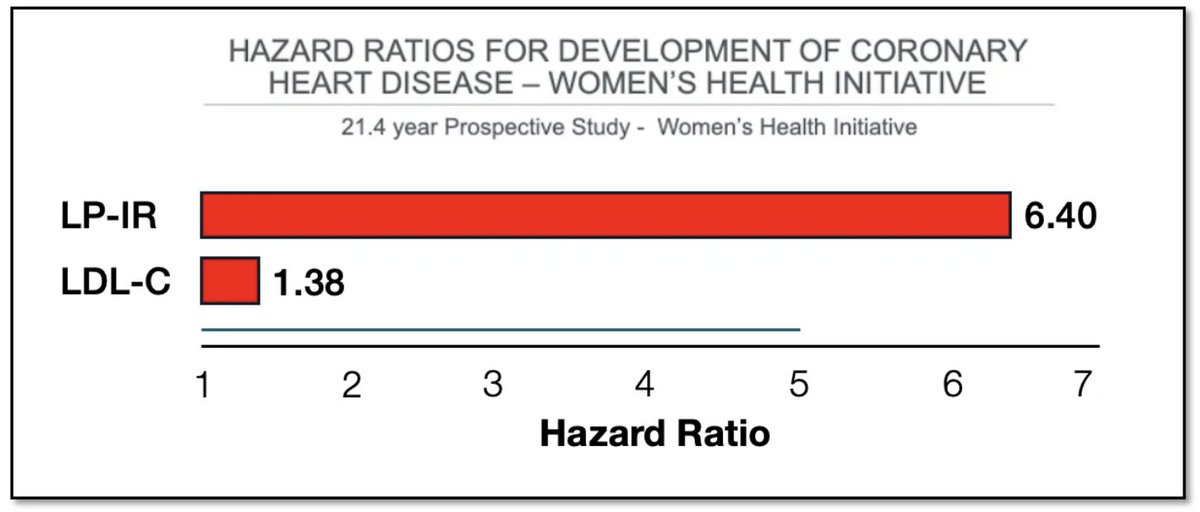
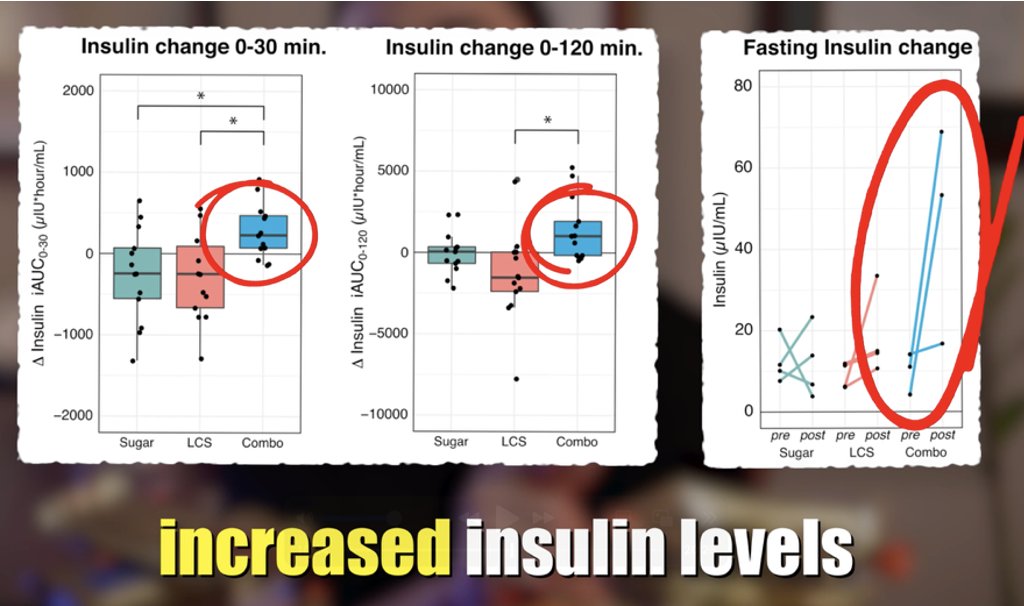
🚨New paper in @Nature reveals link between #microbiome & #exercise motivation!🧠🐁
👉Provides foundational insight that could lead to dietary practices & supplements to promote exercise motivation
Video:
@hubermanlab
#dopamine
1/ Thread🧵👇
👉Provides foundational insight that could lead to dietary practices & supplements to promote exercise motivation
Video:
@hubermanlab
#dopamine
1/ Thread🧵👇

2/ The study in question is a mouse study, as you can really perform these fine mechanistic study in animal models
Data show that genetics variation among mice was a minor contributor and the variations in microbiome composition were more important for physical performance...
Data show that genetics variation among mice was a minor contributor and the variations in microbiome composition were more important for physical performance...

3/ To show the microbiome mediates the effects, they knocked out the microbiome with antibiotics in high-performer mice and the result was an impairment physical performance by 50%! (2a)
By contrast, microbiome transplant could enhance performance.
By contrast, microbiome transplant could enhance performance.

4/ Progressed to test hypothesis that motivation to exercise accounted for effect of microbiome on physical performance
Found that exercise ⬆️ dopamine levels (controls motivational state) AND exercise-induced ⬆️ in DA could be blunted w/ antibiotics to destroy microbiome
Found that exercise ⬆️ dopamine levels (controls motivational state) AND exercise-induced ⬆️ in DA could be blunted w/ antibiotics to destroy microbiome

5/ Figure shows DA levels are NOT impacted in the basal state w/ antibiotic treatment but that antibiotics do prevent the rise in DA following exercise – consistent with the possibility that microbiome dysfunctioncould impact motivational states related to exercise
@hubermanlab
@hubermanlab

6/ Zooming fwd so as not to get tedious, by blocking elements of pathways or activating elements, the team demonstrated there is an axis whereby certain bugs produce metabolites that activate neurons that signal to DA motivational centers in the brain to want to exercise
7/ Specifically, the most potent gut derived metabolites were fatty acid amines, such as N-oleoylethanolamide (OEA) (5e), which – for the super nerds out there – is a lipid that acts on the endocannabinoid pathway and is structured based on oleic acid, a monounsaturated fat 

8/ The scientists were even able to show that gastric infusions of (OEA) recapitulated the effects of dopamine increase and improved exercise motivation/performance, and that more OEA correlated with more running (5g) 

9/ These data spell out a story showing a strong link between the microbiome and exercise motivation
By better understanding these pathways, we could create dietary protocols or probiotics that could make us want to move our bodies more, improving personal and public health
By better understanding these pathways, we could create dietary protocols or probiotics that could make us want to move our bodies more, improving personal and public health
10/ Plug again for 5 min video overview & Happy Saturday!
• • •
Missing some Tweet in this thread? You can try to
force a refresh