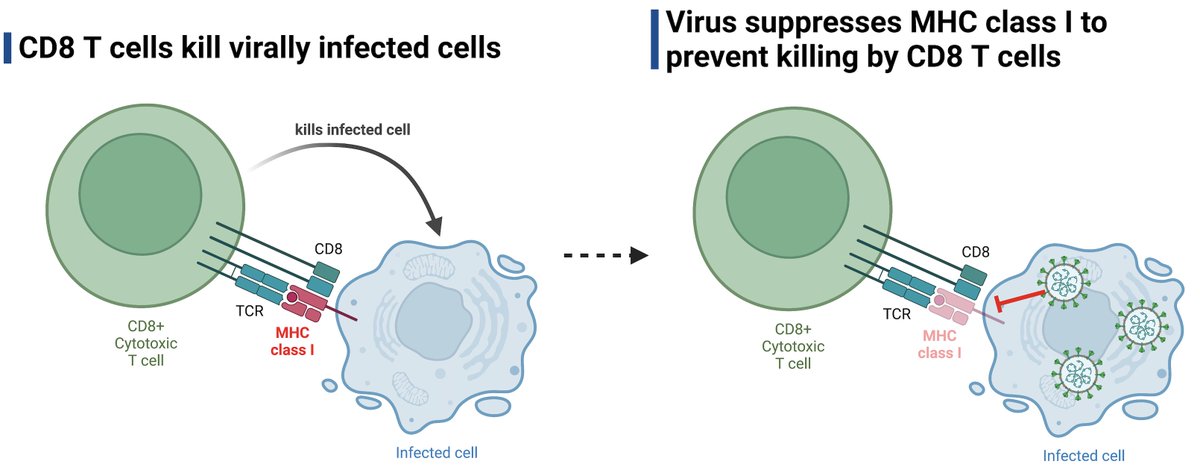
A while ago, @MiyuMoriyama et al showed that SARS-CoV-2 variants suppress MHC I levels in infected cells to the same degree as the ancestral virus. Then came the Omicron variants. A short update. (1/) biorxiv.org/content/10.110…
https://twitter.com/VirusesImmunity/status/1522939444856193024
Since the original submission, @MiyuMoriyama, with the help of @NathanGrubaugh's team & @carolilucas, obtained and analyzed the ability of Omicron subvariants shown here 👇🏽 Miyu gated on spike-positive (infected) cells and compared MHC I levels to uninfected (S-) cells. (2/) 

Note that MHC I surface levels are only downregulated in the infected (S+) cells, but not in the uninfected cells (S-) in the same tissue culture wells. SARS-CoV-2 evades recognition of the infected cells by cytotoxic T cells but has no impact on the surrounding cells. (3/)
New data are pretty striking. Compared to the ancestral (WA1) or Epsilon (B.1.429) variant, Omicron subvariants BA.1, BA.2.12.1, XAF and BA.4 all suppressed MHC I surface expression better in infected cells. An irrelevant surface marker (CD324) was not affected by infection. (4/) 

Our new data demonstrate the evolution of the Omicron variants in further suppressing MHC I expression and escaping detection/killing by CD8 T cells. Thus, in addition to evasion from Ab and innate immunity, MHC I ⬇️ by Omicron may further ⬆️ replication & transmission. (End)
• • •
Missing some Tweet in this thread? You can try to
force a refresh