1/18) 🚨NEW PAPER in @AJCN provides evidence supporting #CIM of #obesity
👉 Glycemic Load >>> Calorie Counting for weight loss
👉 Biomarkers of Low carb diet predict weight
👉Insulin hyper-secreters especially benefit fromlow-carb
Video 👉👉
🧵...
👉 Glycemic Load >>> Calorie Counting for weight loss
👉 Biomarkers of Low carb diet predict weight
👉Insulin hyper-secreters especially benefit fromlow-carb
Video 👉👉
🧵...

2/18) First, and foremost, a big congrats to @AdrianSotoMota and @davidludwigmd for an excellent work!
& of course, for those who have the time, I highly recommend reading the full paper, here: doi.org/10.1016/j.ajcn…
But for those who prefer video or TW thread... HERE WE GO...
& of course, for those who have the time, I highly recommend reading the full paper, here: doi.org/10.1016/j.ajcn…
But for those who prefer video or TW thread... HERE WE GO...
3/18) What the researchers did in this paper is perform a secondary analysis of pre-existing data from a 12-month RCT: the DIETFITS trial in which 609 adults aged 18-50 without diabetes were randomized to either a 12 m Low-carb diet (LCD) or low-fa diet (LFD).
4/18) Initial data showed LCD led to more weight loss than LFD at 3 and 6 mo, but that the between group diff in weight loss lost significance at 12 months
This was taken to be evidence against the CIM. However, as this paper reveals, there is more nuance to the story...
This was taken to be evidence against the CIM. However, as this paper reveals, there is more nuance to the story...

5/18) One important ? is why significance was lost at 12mo?
2 reasons...
i) Participant dropout. Each group lost ~80 participants, diminishing statistic power
AND
ii) Diet convergence: ‘Carb creep’ in the LCD group (132g/d) + carb drop in the LFD group (213g/d)
2 reasons...
i) Participant dropout. Each group lost ~80 participants, diminishing statistic power
AND
ii) Diet convergence: ‘Carb creep’ in the LCD group (132g/d) + carb drop in the LFD group (213g/d)
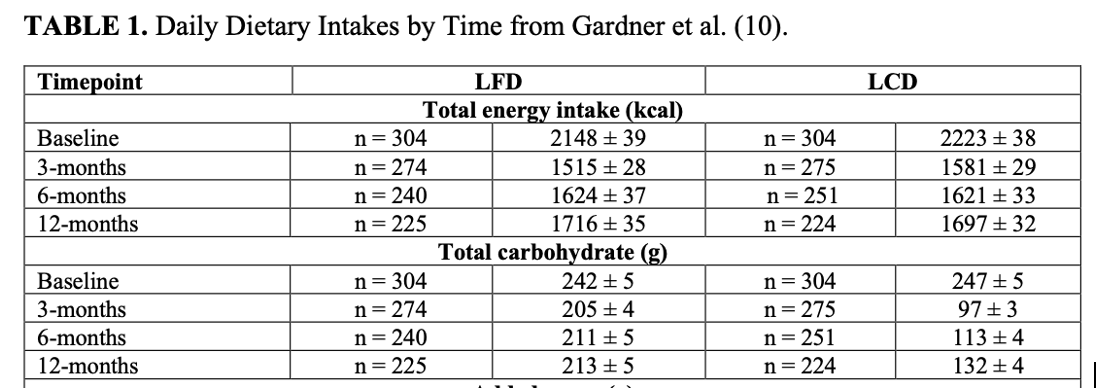
6/18) Even setting aside the dietary convergence, when missing data was imputed, the LCD group did in fact lose more weight at 12 m than the LFD group at all time points. 

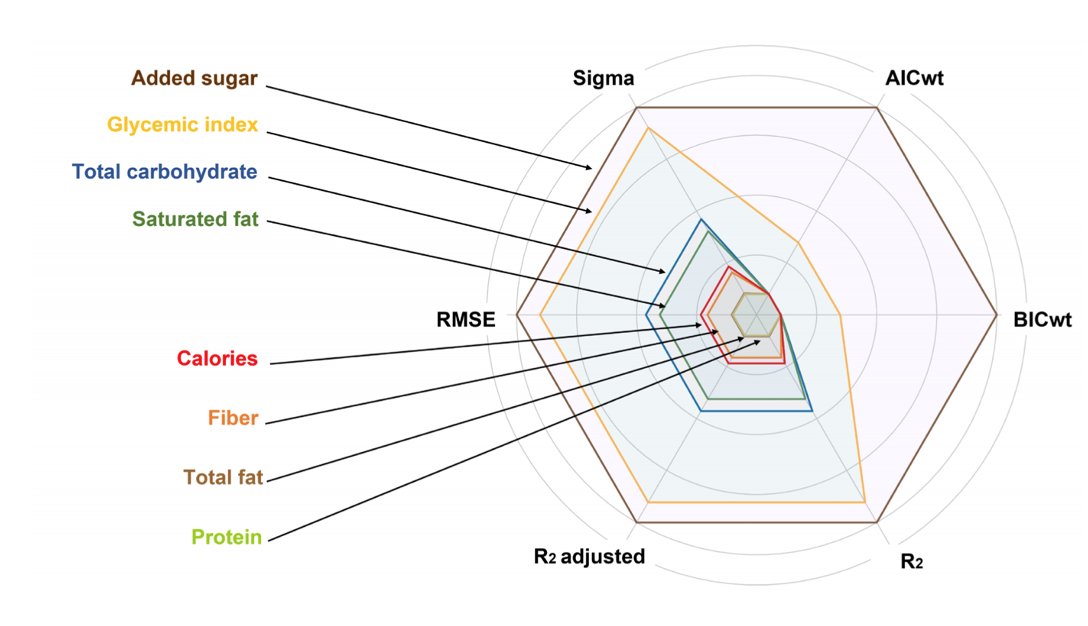
7/18) Moving on, Fig 2 shows a model of predictors of weight loss ➡️ larger circumferences represent better predictors of weight loss
🍽️🍽️Total fat & Calories were poorer predictors of weight loss
🍩🥭Carbs & GI and sugar are the superior predictors
Consistent with the CIM
🍽️🍽️Total fat & Calories were poorer predictors of weight loss
🍩🥭Carbs & GI and sugar are the superior predictors
Consistent with the CIM
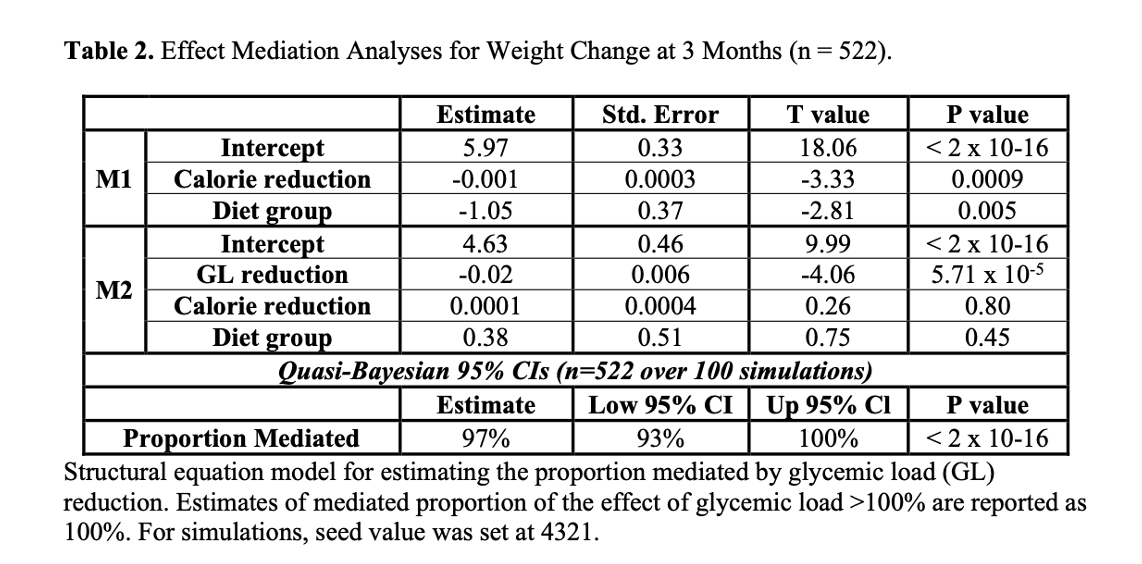
8/18) In Table are 2 models examining the mediators of weight loss
When you add in Glycemic Load (GL) is added to the model in model 2, GL is highly significant (p=5.7x10-5) and calories (p=0.80) LOSE significance!
Let’s expand on this point...
When you add in Glycemic Load (GL) is added to the model in model 2, GL is highly significant (p=5.7x10-5) and calories (p=0.80) LOSE significance!
Let’s expand on this point...
9/18) Many think that LCD works bc it just makes you eat fewer calories, but that it’s actually the drop in calorie intake that’s driving the weight loss. This certainly is a contributing factor but...
10/18) These results show that GL is better than caloric intake at predicting weight loss, which may appear counterintuitive BUT can be made intuitive if you think about the components of Energy balance: Calories in & Calories out...
11/18) The CICO model, when taken in CLINICAL practice, usually focuses on CI because accurately measuring CO accurately is next to impossible (think, NEAT, TEF, body temp, etc.)
12/18) By contrast, if GL influences the hormonal milieu of the body, it dictates NOT ONLY hunger and caloric intake (CI) but also homeostatic mechanisms to maintain energy equilibrium, or tip it one way or another, through CO: NEAT, body temp, etc.
13/18) Simply put, one could actually make the argument that the CIM is actually a superior real-life ‘CICO’ model than the standard cal counting CICO model itself!
LOL If that doesn’t make sense, read ^ again and watch video for completeness
LOL If that doesn’t make sense, read ^ again and watch video for completeness
14/18) Other cool data presented in this paper consistent with the results already shared is that, in fig 5, a biomarker of low-carb/GL (TG/HDL) was strongly associated with weight loss whereas a biomarker of fat reduction (LDL+HDL) was not. 

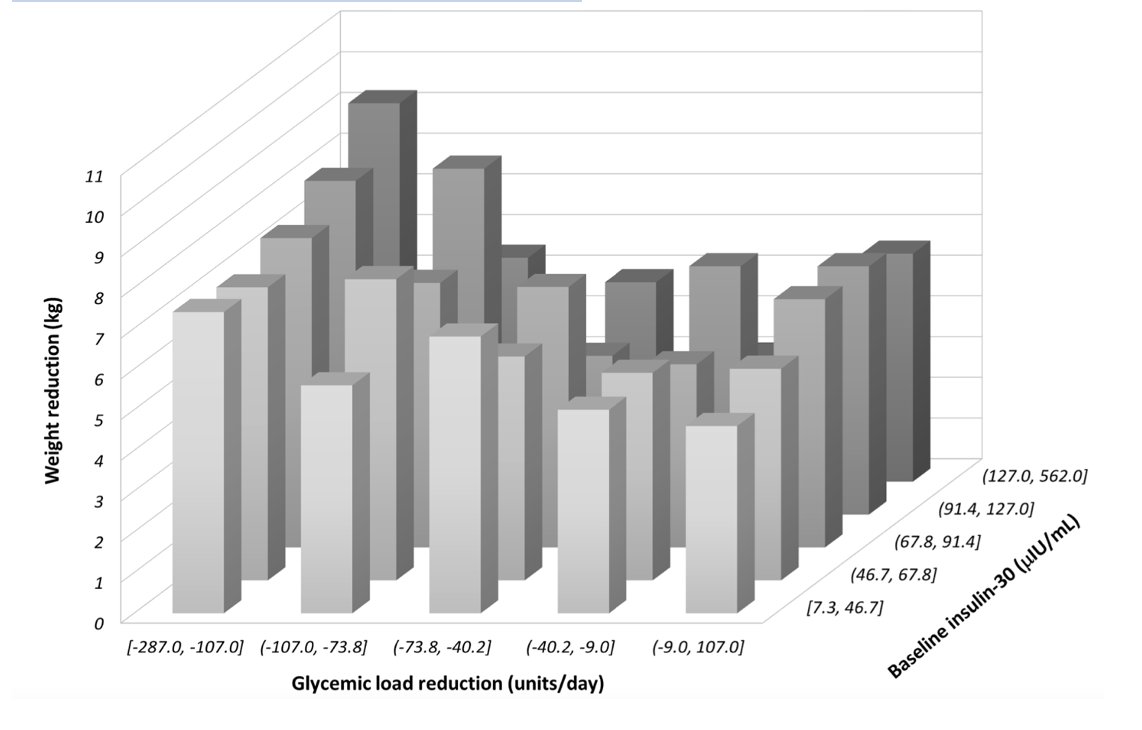
15/18) Finally, and beautifully, the authors put a bold prediction of the CIM to the test which is that those with higher basal insulin secretion would benefit most for GL reduction. Again, this is b/c in the CIM GL influences insulin to cause fat storage.
16/18) So, if someone naturally is an insulin hyper-secreter, the effect of the model is simply going to be amplified. As a result, those who secrete a lot of insulin probably benefit the most from reducing GL. Is that the case? As it turns out yes!
17/18) Clearly see an interaction b/w GL reduction and basal insulin
As you can see in the back left row, these persons who reduced GL most and were the insulin hyper secreters, lost the most weight!
As you can see in the back left row, these persons who reduced GL most and were the insulin hyper secreters, lost the most weight!
18/18) In summary, this paper provides powerful evidence for two prediction of the CIM: (1) GL > Calories as a predictor of weight loss and (2) insulin hyper secreters benefit most from carb reduction.
Video
Paper
doi.org/10.1016/j.ajcn…
Video
Paper
doi.org/10.1016/j.ajcn…
• • •
Missing some Tweet in this thread? You can try to
force a refresh