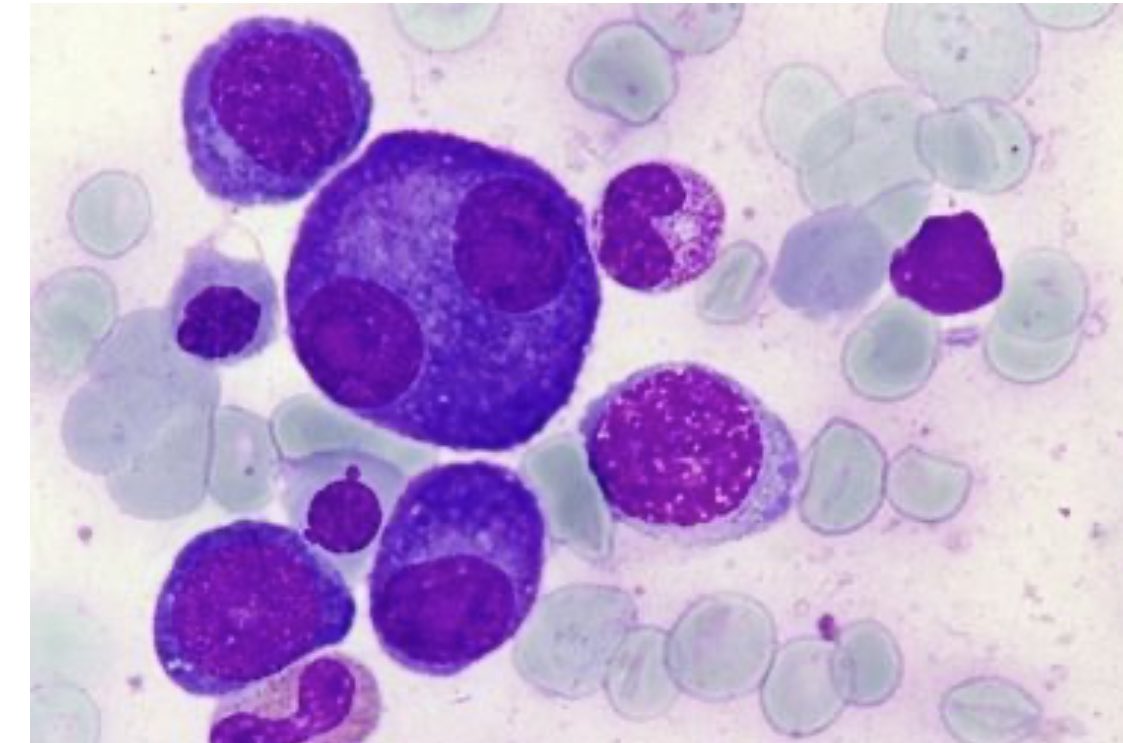
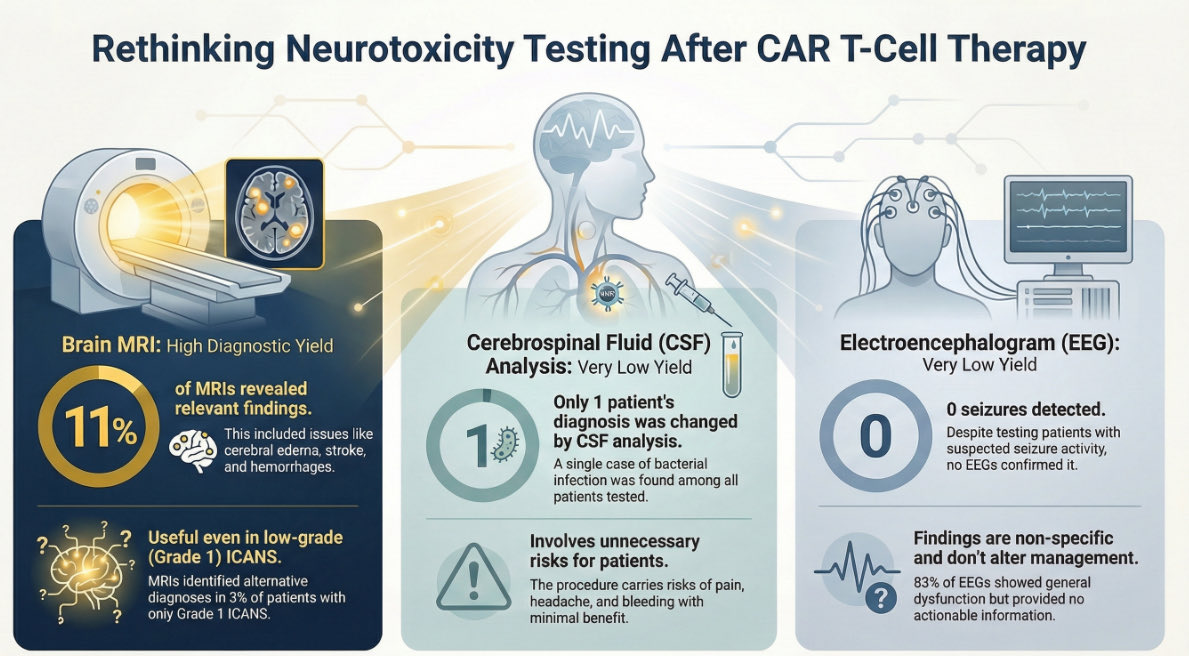
Flame Cells: These are plasma cells with vermillion-staining glycogen-rich overstuffed fibrils. Although these cytoplasmic features are suggestive of neoplastic plasma cells, can also be found in reactive cells. imagebank.hematology.org/image/3230/fla… #mmsm #myeloma #MedTwitter #USMIRC #MedEd 



Mott cell is a plasma cell characterized by an accumulation of multiple Russell bodies, globular cytoplasmic inclusions composed of immunoglobulin imagebank.hematology.org/image/61837/mo… #mmsm #myeloma #MedTwitter #MedEd #USMIRC 


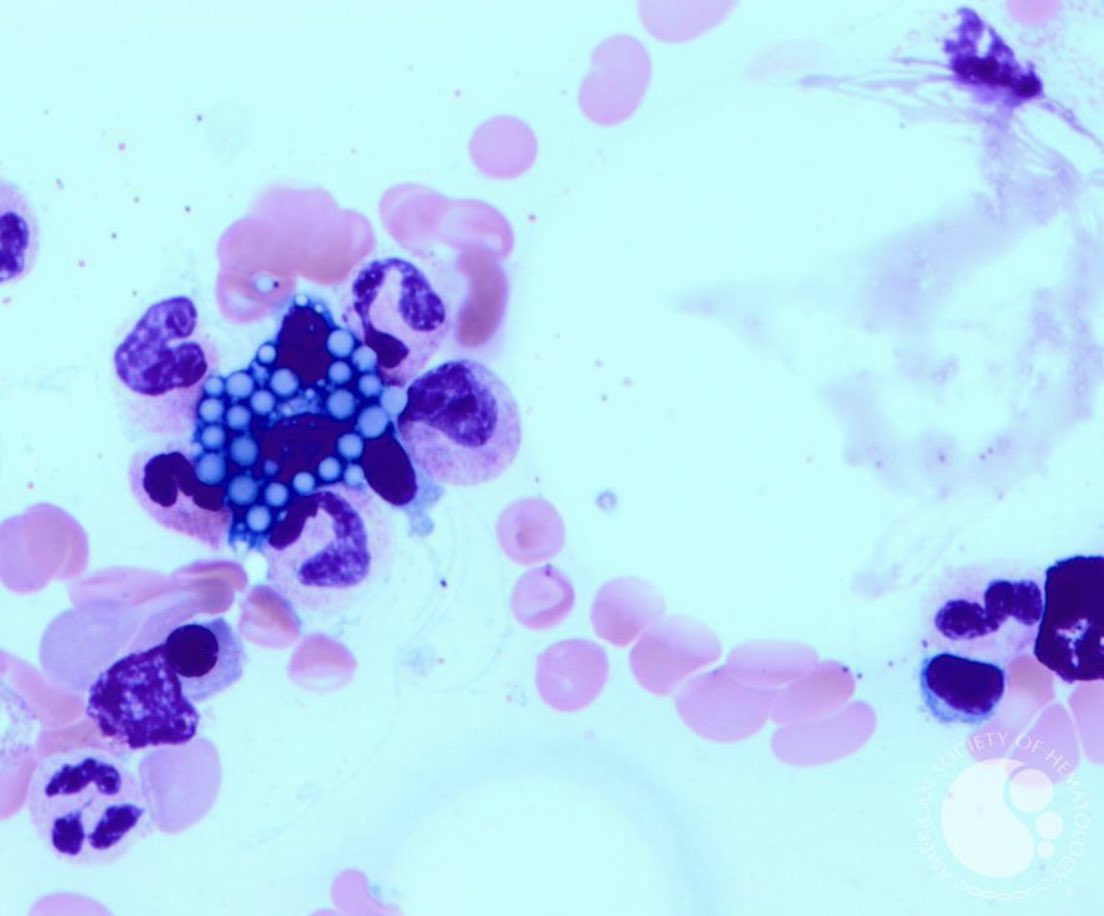
Russell bodies are cytoplasmic inclusions filled with immunoglobulins imagebank.hematology.org/image/4192/rus… #mmsm #myeloma #MedTwitter #MedEd #USMIRC 



Dutcher bodies (arrow) are intranuclear inclusions found in plasma cells. imagebank.hematology.org/image/1073/dut… #mmsm #myeloma #MedTwitter #MedEd #USMIRC 



inclusion bodies in plasma cells have been repeatedly reported, mainly concentrated in the Auer rod-like inclusions link.springer.com/article/10.100… #mmsm #myeloma #MedTwitter #MedEd #USMIRC 



• • •
Missing some Tweet in this thread? You can try to
force a refresh