
Associate Professor,Plasma Cell disorder program Director, Division of HMCT/University of Kansas Medical Center, no COI, #USMIRC Founder, #mmsm @KUMedCenter
How to get URL link on X (Twitter) App

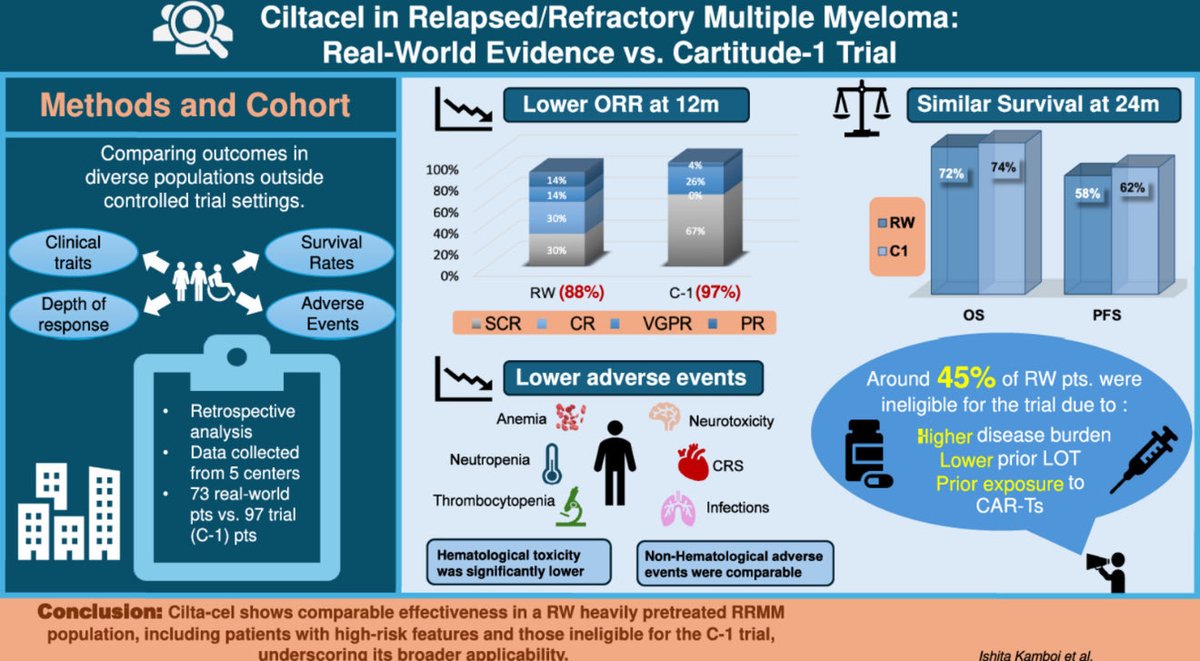
 📊 Study Design
📊 Study Design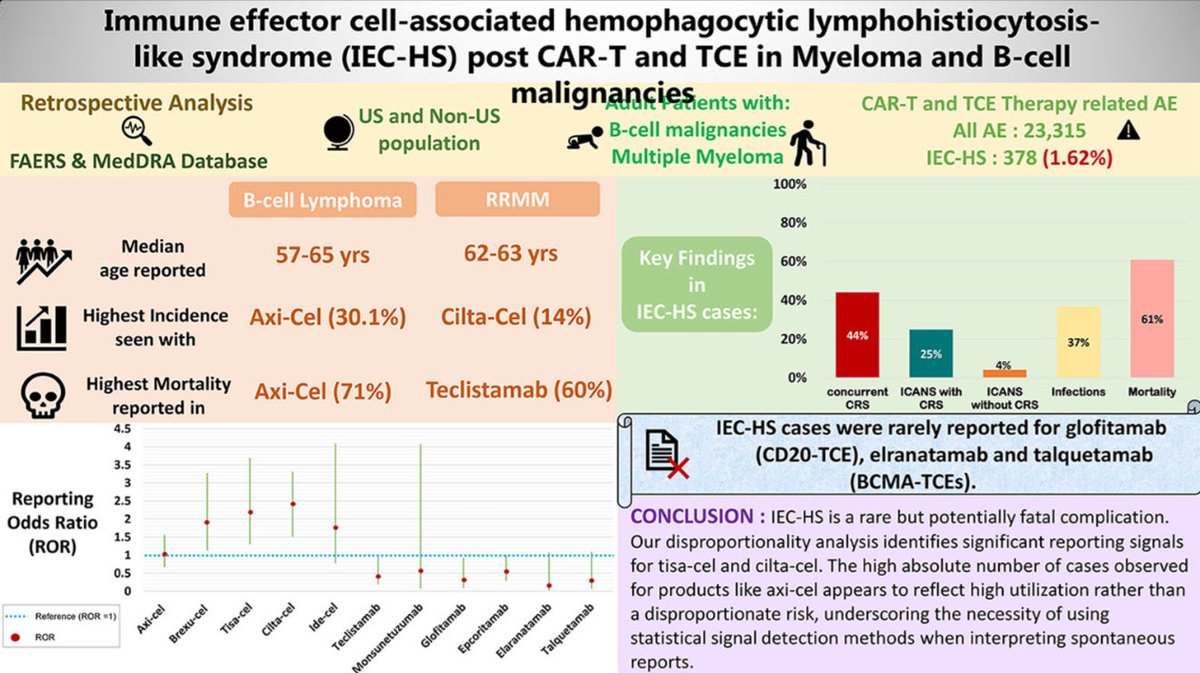
 2️⃣ Who reports it most?
2️⃣ Who reports it most?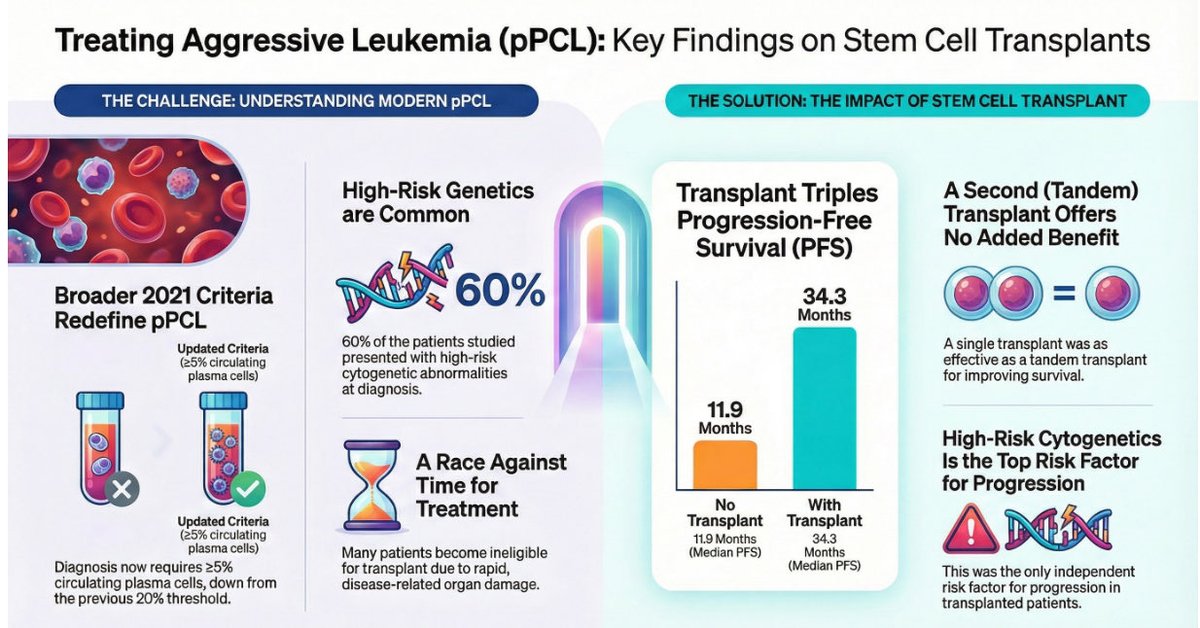
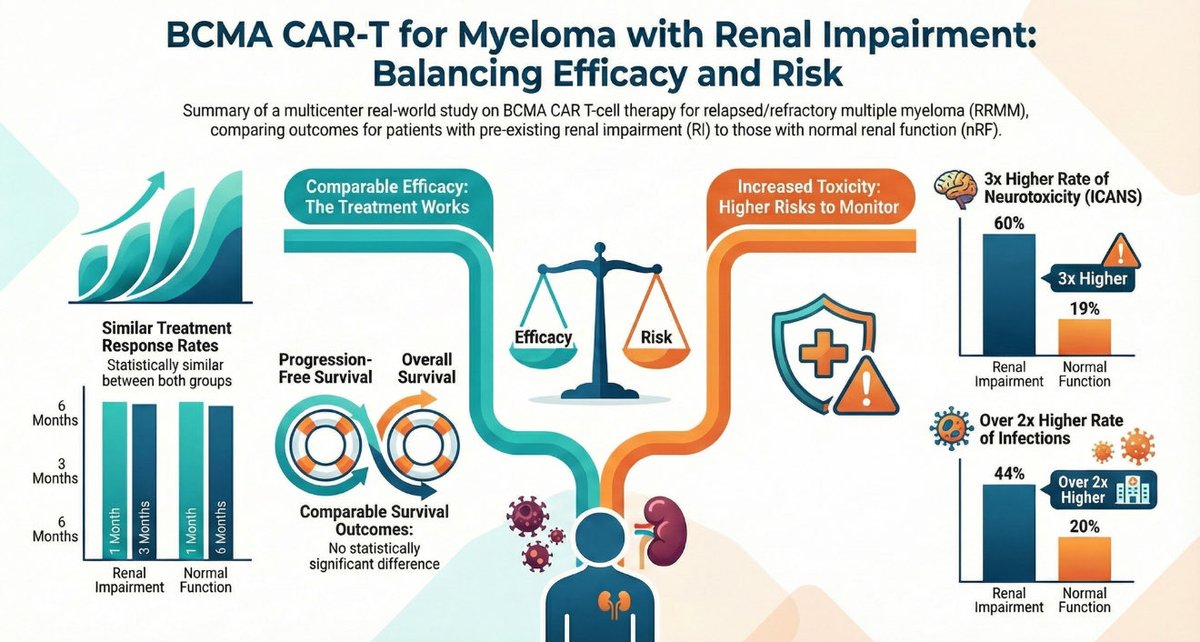
 2️⃣ Study snapshot 🧪
2️⃣ Study snapshot 🧪
 2/ Background 🧬
2/ Background 🧬
 2/Core Finding
2/Core Finding
 1️⃣ Why spleen size?
1️⃣ Why spleen size?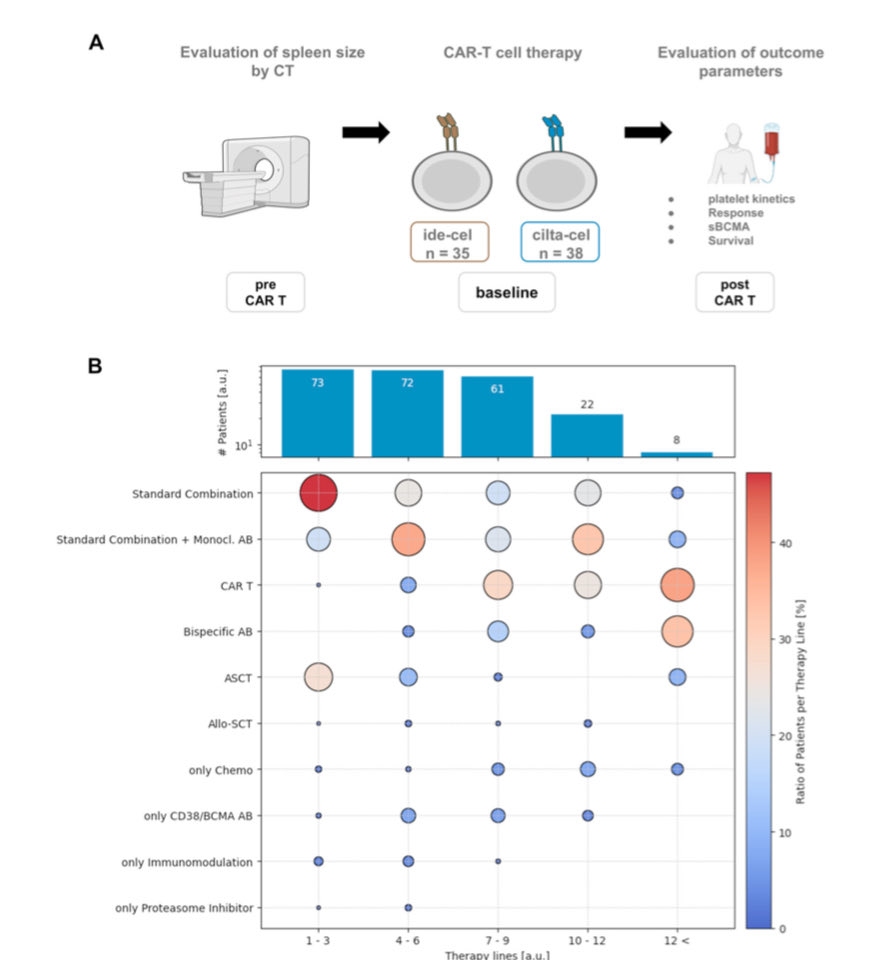
 2️⃣ Baseline Differences
2️⃣ Baseline Differences
 2/Multiple Myeloma trials: Industrial trials constitute the largest proportion of MM trials (40%), followed closely by AS trials (37%).
2/Multiple Myeloma trials: Industrial trials constitute the largest proportion of MM trials (40%), followed closely by AS trials (37%). 

 2/🌡️ Characteristics of Cilta-cel-associated Parkinsonism: The onset is typically subacute, emerging approximately 1-2 months after CAR T-cell infusion.
2/🌡️ Characteristics of Cilta-cel-associated Parkinsonism: The onset is typically subacute, emerging approximately 1-2 months after CAR T-cell infusion.
 2/ ⚜️Study Design: ISB 2001 is administered weekly SC with a step-up dosing strategy (D1 & D4) followed by the full target dose (D8 onwards).
2/ ⚜️Study Design: ISB 2001 is administered weekly SC with a step-up dosing strategy (D1 & D4) followed by the full target dose (D8 onwards).
 2/Treatment: Single IV dose of anti-GPRC5D CAR T cells at 2 × 10⁶ cells per kg.
2/Treatment: Single IV dose of anti-GPRC5D CAR T cells at 2 × 10⁶ cells per kg.


 2/High-Risk Patient Population:♣️
2/High-Risk Patient Population:♣️


 2/ The introduction of elranatamab is projected to increase total costs by $553,607 over three years, resulting in a per member per month (PMPM) increase of $0.05.
2/ The introduction of elranatamab is projected to increase total costs by $553,607 over three years, resulting in a per member per month (PMPM) increase of $0.05.

 2/Auto-HCT demonstrates improved overall survival compared to historical outcomes, but is limited by high relapse rates. GO TO POST 4 ♦️
2/Auto-HCT demonstrates improved overall survival compared to historical outcomes, but is limited by high relapse rates. GO TO POST 4 ♦️
 2/⏳123 eligible patients with intermediate/high-risk SMM were randomized (1:1:1) to 3 intravenous (IV) daratumumab 16 mg/kg dosing schedules: long-intense, intermediate, and short-intense. 🛣️
2/⏳123 eligible patients with intermediate/high-risk SMM were randomized (1:1:1) to 3 intravenous (IV) daratumumab 16 mg/kg dosing schedules: long-intense, intermediate, and short-intense. 🛣️

 Many differential diagnosis; mostly dependent on clinical diagnosis:
Many differential diagnosis; mostly dependent on clinical diagnosis: 

