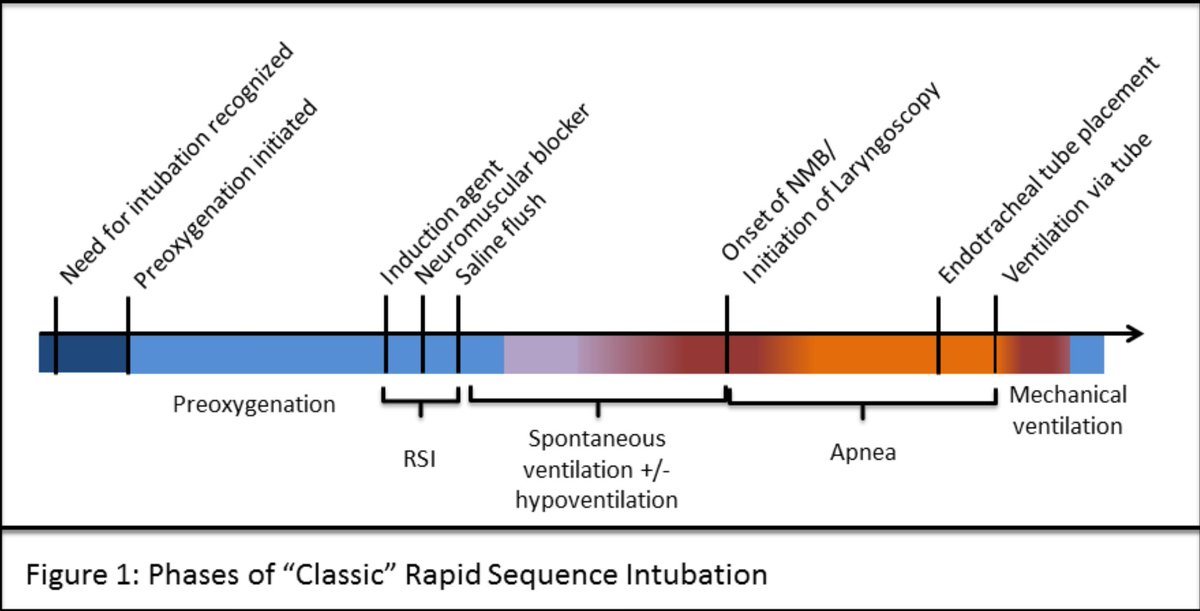
Determination of the Society for Cardiovascular Angiography and Intervention (SCAI) Shock stage using the revised SCAI Shock Classification 

Conceptual model showing the overlap between
different states of hemodynamic compromise. Shock is defined by presence of hypoperfusion; most, but NOT ALL, patients will also be hypotensive. Pts w hemodynamic instability who do not meet criteria for shock are labeled as pre-shock
different states of hemodynamic compromise. Shock is defined by presence of hypoperfusion; most, but NOT ALL, patients will also be hypotensive. Pts w hemodynamic instability who do not meet criteria for shock are labeled as pre-shock

Management algorithm for patients with or at risk for cardiogenic shock (CS) tailored to the Society for Cardiovascular Angiography and Intervention (SCAI) Shock stage 

Framework of clinical parameters to follow in patients with heart failure-related cardiogenic shock in the critical care unit 

It seems that the most controversial issue is the use of short-term mechanical circulatory support for cardiogenic shock. So, a very recent publication deals with this: 

Flowchart to identify and handle potential need for escalation of mechanical circulatory support in patients supported by axial flow pump (AFP) 

• • •
Missing some Tweet in this thread? You can try to
force a refresh