
Physician, epidemiologist & clinical trialist: https://t.co/PuSJzZsPC6 @oxford_ndph. Lead, Good Clinical Trials Collaborative https://t.co/Do4H97gzf0. CEO https://t.co/4YkPhZtWPG
How to get URL link on X (Twitter) App




 6609 pts with CKD (not requiring RRT)
6609 pts with CKD (not requiring RRT) 

 Some will ask, why the study was so big.
Some will ask, why the study was so big.
 Our approach - Quality by Design:
Our approach - Quality by Design: 






 In post-hoc exploratory analyses.
In post-hoc exploratory analyses. 

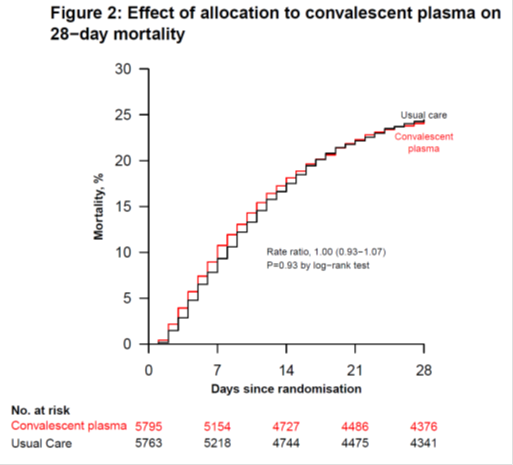
 No improvement in survival with convalescent plasma, regardless of patient antibody status at baseline:
No improvement in survival with convalescent plasma, regardless of patient antibody status at baseline: 

 Benefits seen in wide range of patients (simple oxygen... mechanical ventilation)
Benefits seen in wide range of patients (simple oxygen... mechanical ventilation) 
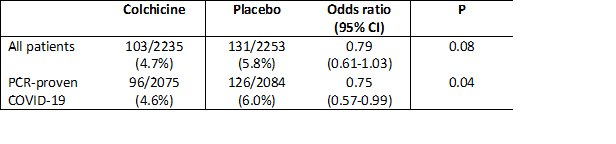


 This is an important result:
This is an important result:


 Similar results in different age-groups and whether on mechanical ventilation or not:
Similar results in different age-groups and whether on mechanical ventilation or not: 
https://twitter.com/FinancialTimes/status/1316822064427339776Doubtless there will be much discussion, not all of which will be as objective as we need in a pandemic.

 1561 patients were randomised to HCQ + usual care
1561 patients were randomised to HCQ + usual care
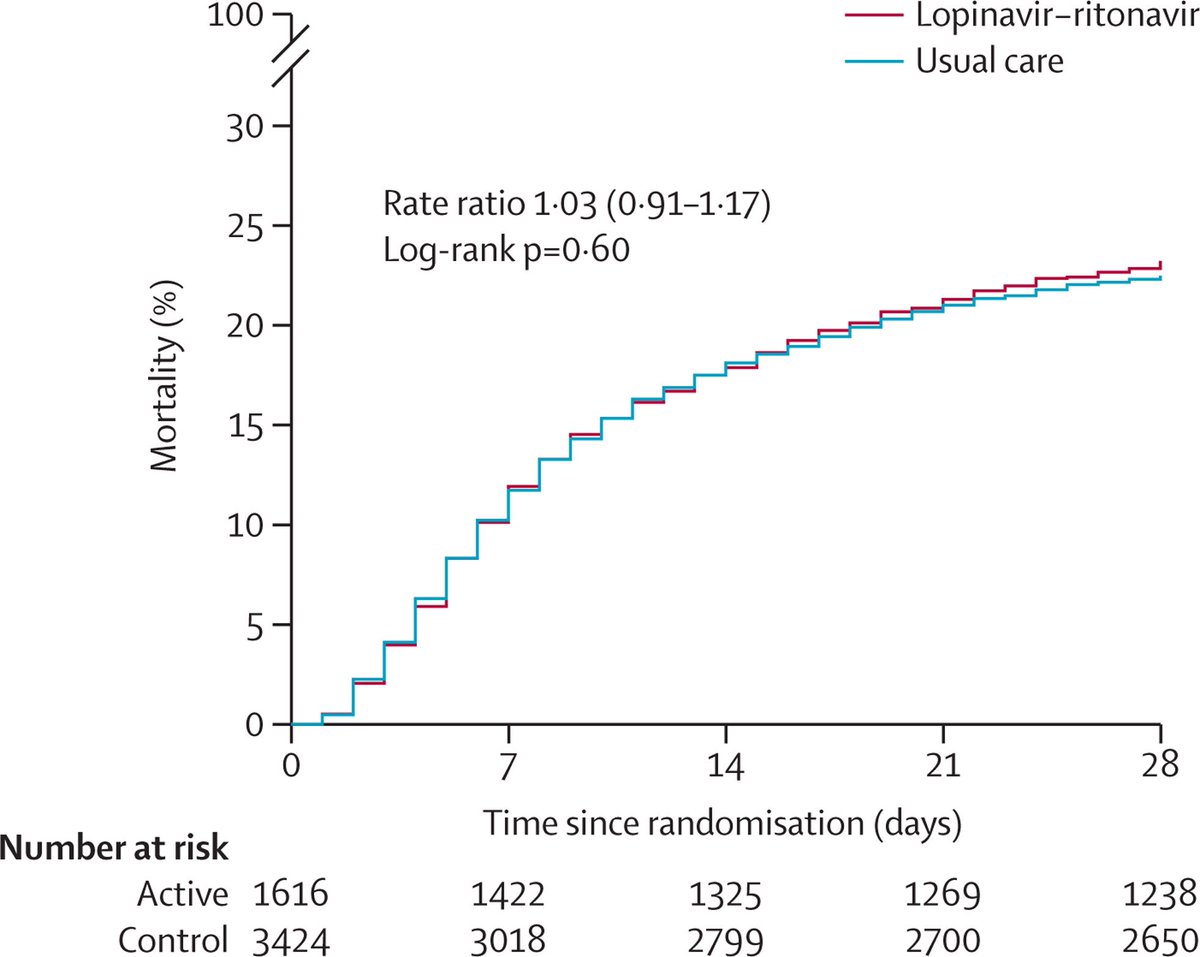
 Lopinavir-ritonavir is an HIV treatment that was widely recommended and used for #COVID19. The RECOVERY trial has shown it does not work - so doctors no longer use it.
Lopinavir-ritonavir is an HIV treatment that was widely recommended and used for #COVID19. The RECOVERY trial has shown it does not work - so doctors no longer use it.