
🔬Diving deep into #MECFS, #LongCOVID, #LongEBV & post-vaccine syndrome research. Dual role:Researcher and EBV ME/CFS patient. Seeking answers. #Antivirals #HLA
How to get URL link on X (Twitter) App


 1) Why does anti-M3 matter?
1) Why does anti-M3 matter?
 1)🔴 New evidence
1)🔴 New evidencehttps://twitter.com/vipintukur/status/2019803195103674764

 2️⃣ Don’t feel ashamed to:
2️⃣ Don’t feel ashamed to:
 📢 𝐖𝐡𝐲 𝐕𝐚𝐜𝐜𝐢𝐧𝐞𝐬 𝐂𝐚𝐧 𝐃𝐞𝐯𝐞𝐥𝐨𝐩 𝐏𝐚𝐭𝐡𝐨𝐥𝐨𝐠𝐢𝐞𝐬 𝐒𝐢𝐦𝐢𝐥𝐚𝐫 𝐭𝐨 𝐏𝐞𝐫𝐬𝐢𝐬𝐭𝐞𝐧𝐭 𝐂𝐎𝐕𝐈𝐃 𝐚𝐧𝐝 𝐏𝐨𝐬𝐭-𝐈𝐧𝐟𝐞𝐜𝐭𝐢𝐨𝐮𝐬 𝐌𝐄/𝐂𝐅𝐒? 📢
📢 𝐖𝐡𝐲 𝐕𝐚𝐜𝐜𝐢𝐧𝐞𝐬 𝐂𝐚𝐧 𝐃𝐞𝐯𝐞𝐥𝐨𝐩 𝐏𝐚𝐭𝐡𝐨𝐥𝐨𝐠𝐢𝐞𝐬 𝐒𝐢𝐦𝐢𝐥𝐚𝐫 𝐭𝐨 𝐏𝐞𝐫𝐬𝐢𝐬𝐭𝐞𝐧𝐭 𝐂𝐎𝐕𝐈𝐃 𝐚𝐧𝐝 𝐏𝐨𝐬𝐭-𝐈𝐧𝐟𝐞𝐜𝐭𝐢𝐨𝐮𝐬 𝐌𝐄/𝐂𝐅𝐒? 📢

 1/La diversidad de microbiota varía entre personas, lo que hace imposible obtener resultados consistentes de los test de microbiota. No se sabe cuál es la microbiota normal. #Microbiota #SIBO #LongEBV #microE2324
1/La diversidad de microbiota varía entre personas, lo que hace imposible obtener resultados consistentes de los test de microbiota. No se sabe cuál es la microbiota normal. #Microbiota #SIBO #LongEBV #microE2324

 𝐃𝐨𝐞𝐬 𝐭𝐡𝐢𝐬 𝐦𝐨𝐝𝐞𝐥 𝐞𝐱𝐩𝐥𝐚𝐢𝐧 𝐭𝐡𝐞 𝐨𝐜𝐜𝐮𝐫𝐫𝐞𝐧𝐜𝐞 𝐨𝐟 𝐝𝐢𝐠𝐞𝐬𝐭𝐢𝐯𝐞 𝐩𝐫𝐨𝐛𝐥𝐞𝐦𝐬, 𝐟𝐨𝐨𝐝 𝐢𝐧𝐭𝐨𝐥𝐞𝐫𝐚𝐧𝐜𝐞𝐬 𝐚𝐧𝐝 𝐚𝐥𝐭𝐞𝐫𝐚𝐭𝐢𝐨𝐧𝐬 𝐢𝐧 𝐭𝐡𝐞 𝐦𝐢𝐜𝐫𝐨𝐛𝐢𝐨𝐭𝐚 𝐢𝐧 𝐭𝐡𝐞𝐬𝐞 𝐩𝐚𝐭𝐢𝐞𝐧𝐭𝐬?
𝐃𝐨𝐞𝐬 𝐭𝐡𝐢𝐬 𝐦𝐨𝐝𝐞𝐥 𝐞𝐱𝐩𝐥𝐚𝐢𝐧 𝐭𝐡𝐞 𝐨𝐜𝐜𝐮𝐫𝐫𝐞𝐧𝐜𝐞 𝐨𝐟 𝐝𝐢𝐠𝐞𝐬𝐭𝐢𝐯𝐞 𝐩𝐫𝐨𝐛𝐥𝐞𝐦𝐬, 𝐟𝐨𝐨𝐝 𝐢𝐧𝐭𝐨𝐥𝐞𝐫𝐚𝐧𝐜𝐞𝐬 𝐚𝐧𝐝 𝐚𝐥𝐭𝐞𝐫𝐚𝐭𝐢𝐨𝐧𝐬 𝐢𝐧 𝐭𝐡𝐞 𝐦𝐢𝐜𝐫𝐨𝐛𝐢𝐨𝐭𝐚 𝐢𝐧 𝐭𝐡𝐞𝐬𝐞 𝐩𝐚𝐭𝐢𝐞𝐧𝐭𝐬?

 Este modelo explica la aparición de problemas digestivos, intolerancias alimentarias y alteraciones en la microbiota en estos pacientes?
Este modelo explica la aparición de problemas digestivos, intolerancias alimentarias y alteraciones en la microbiota en estos pacientes?
https://twitter.com/VirusesImmunity/status/17063329657922727221/ Many wonder why EBV is the main trigger for ME/CFS and Long COVID symptoms, over other factors such as other infections and metal poisoning. Let's dive into it, summarizing our recent review article. Link: link.springer.com/article/10.118…

 1/ 🤔 𝐈𝐧𝐭𝐫𝐨𝐝𝐮𝐜𝐭𝐢𝐨𝐧: ME/CFS and Long COVID are two pathologies that, although arising from different causes, present astonishing similarities in how they affect the body and their symptoms. #MyalgicEncephalomyelitis #COVIDSurvivors
1/ 🤔 𝐈𝐧𝐭𝐫𝐨𝐝𝐮𝐜𝐭𝐢𝐨𝐧: ME/CFS and Long COVID are two pathologies that, although arising from different causes, present astonishing similarities in how they affect the body and their symptoms. #MyalgicEncephalomyelitis #COVIDSurvivors
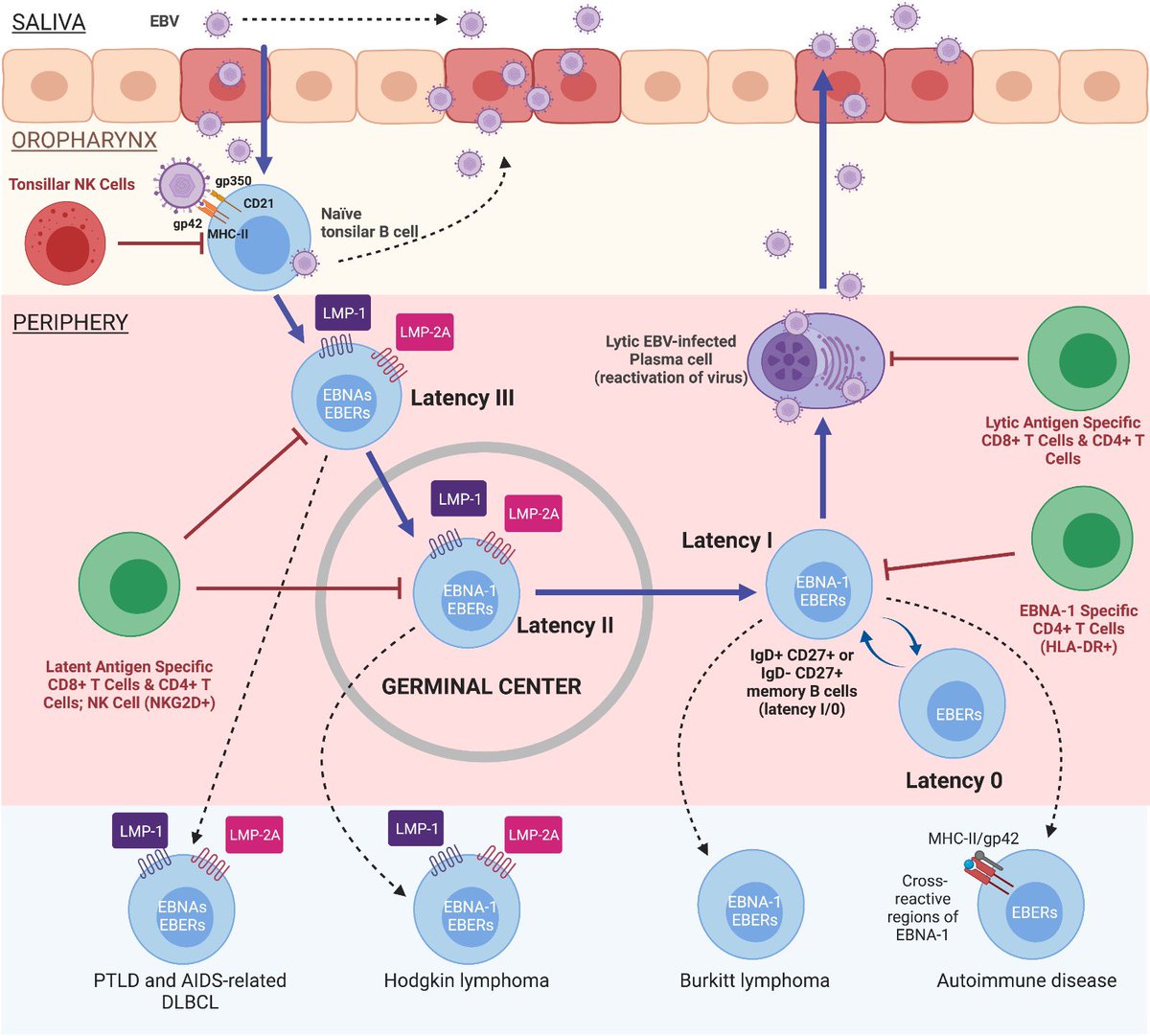
 …Myalgic Encephalomyelitis and Long COVID. But it is still unclear what pathways it uses.
…Myalgic Encephalomyelitis and Long COVID. But it is still unclear what pathways it uses. https://twitter.com/lightup_thepath/status/1551642499604692994Hay que realizar la prueba en lugares donde haya acumulación de estas células, es decir, en tejido, por ejemplo, en la mucosa intestinal. (2)