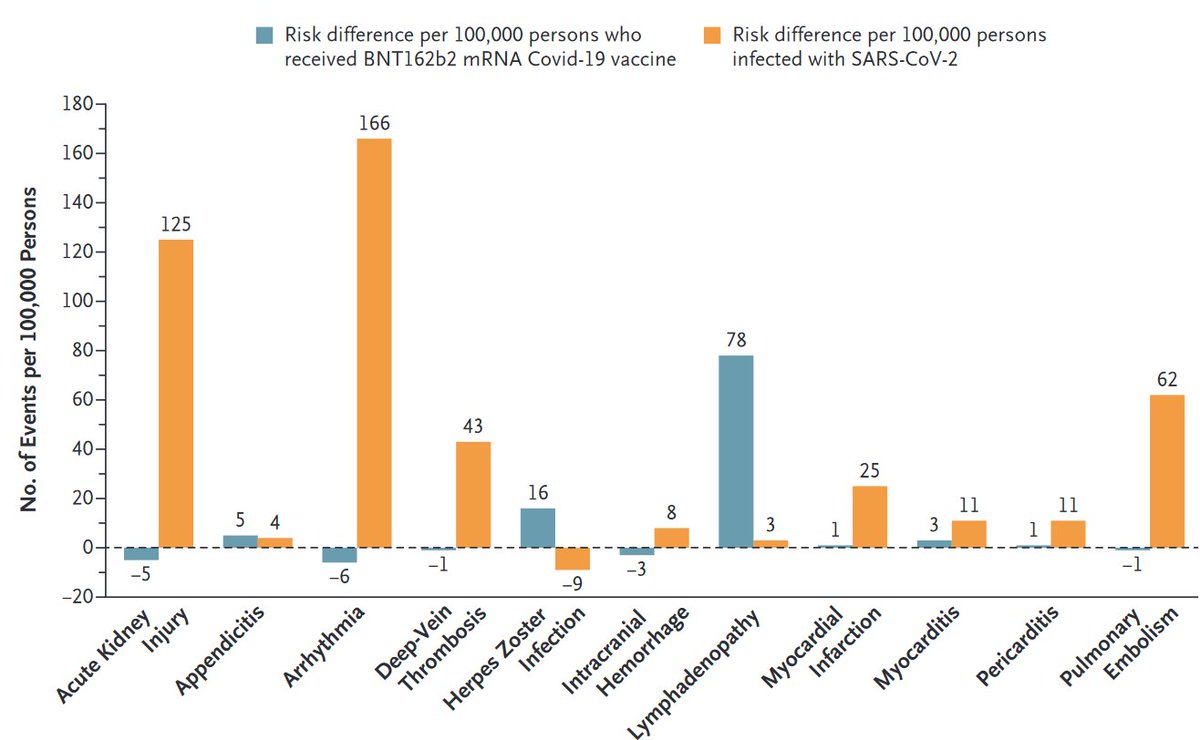
1/
Suppose you want to extend causal inferences from a randomized trial to a target population.
Is that #transportability or #generalizability?
Issa Dahabreh and I propose an answer in this brief commentary in the European Journal of Epidemiology:
ncbi.nlm.nih.gov/pubmed/31218483
Suppose you want to extend causal inferences from a randomized trial to a target population.
Is that #transportability or #generalizability?
Issa Dahabreh and I propose an answer in this brief commentary in the European Journal of Epidemiology:
ncbi.nlm.nih.gov/pubmed/31218483

2/
For those interested in methods for extending inferences from randomized trials to a target population:
Take a look at our tutorial
arxiv.org/pdf/1805.00550…
(soon to appear in Statistics in Medicine)
You will find identification conditions AND three estimation approaches.
For those interested in methods for extending inferences from randomized trials to a target population:
Take a look at our tutorial
arxiv.org/pdf/1805.00550…
(soon to appear in Statistics in Medicine)
You will find identification conditions AND three estimation approaches.

3/
Want more?
This article in @Biometrics_ibs considers estimators to generalize inferences from individuals in randomized trials to all trial-eligible individuals:
ncbi.nlm.nih.gov/pubmed/30488513
And this article in @EpidemiologyLWW clears some confusions:
journals.lww.com/epidem/fulltex…
Want more?
This article in @Biometrics_ibs considers estimators to generalize inferences from individuals in randomized trials to all trial-eligible individuals:
ncbi.nlm.nih.gov/pubmed/30488513
And this article in @EpidemiologyLWW clears some confusions:
journals.lww.com/epidem/fulltex…
@Biometrics_ibs @EpidemiologyLWW 4/
Do you—like @yudapearl below—prefer to express your #generalizability assumptions using causal diagrams?
No problem. Led by Issa Dahabreh, here
arxiv.org/abs/1906.10792
we use graphs to examine the conditions for generalizability of causal inferences from a #randomized trial.
Do you—like @yudapearl below—prefer to express your #generalizability assumptions using causal diagrams?
No problem. Led by Issa Dahabreh, here
arxiv.org/abs/1906.10792
we use graphs to examine the conditions for generalizability of causal inferences from a #randomized trial.

5/
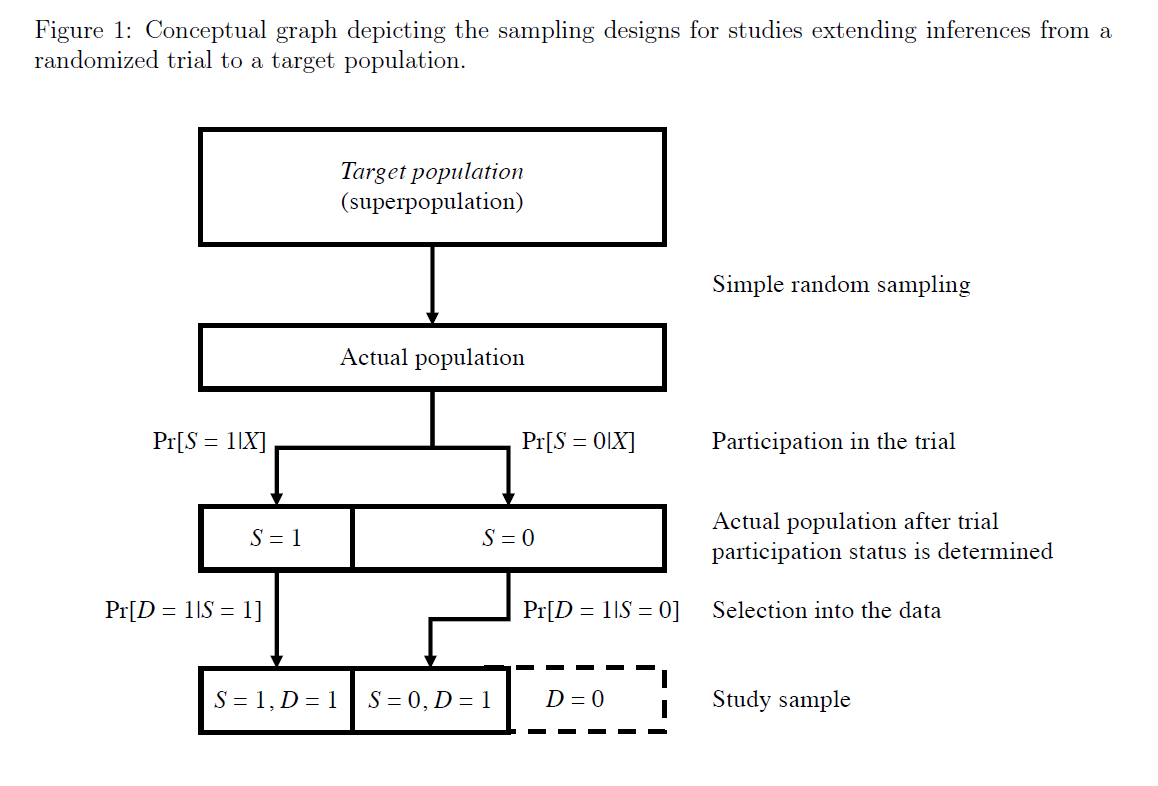
Also, so much talk about extending results from #randomizedtrials to a target population.
But so little guidance on how to design data collection.
Here we provide a unified description of study designs for #transportability and #generalizability.
pubmed.ncbi.nlm.nih.gov/33324969/
Also, so much talk about extending results from #randomizedtrials to a target population.
But so little guidance on how to design data collection.
Here we provide a unified description of study designs for #transportability and #generalizability.
pubmed.ncbi.nlm.nih.gov/33324969/
• • •
Missing some Tweet in this thread? You can try to
force a refresh