A staggering 42% achieved clinical benefit in a population that had exhausted all other methods of disease/cancer treatment
For a population which has been resistant to all other lines of therapies, this is hugely encouraging... if they can treat the most resistant...

...cancers in terminally ill patients, this bodes EXTREMELY well for earlier lines of therapy😬
Also, as the patient population has no other effective treatment this significantly increases the chances of early approval off the back of Phase 2 data
Potentially FDA...
...approval within 2 years
I have already discussed how treating only terminally ill patients with Lung and Kidney Cancer could result in Hundreds of Millions of revenue per annum💰💵
In Phase 2 (expanded patient population) I have explained why I now expect the...
... Efficacy of the combination to increase, further validating this therapy for the treatment of Lung and Kidney Cancer
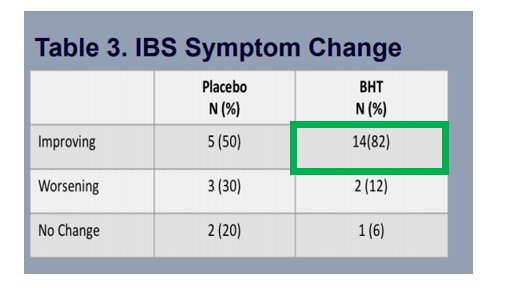
As you can see below 3/12 of the patients withdrew far too early into the trial with an additional 2 also withdrawing early
The Fact that the therapy has seen no Serious Side Effects in conjunction with this ever growing body of data suggest this combination will be clinically and FDA approved and on the market for GLOBAL sales very shortly🌎🗺️
and Finally...

Professor Jaap Verweij shares my EXCITEMENT how data which is slowly unfolding is showing a truly novel approach @4dpharmaplc has to Cancer treatment and regulation of the body immune system
'Extremely Encouraging' is the professional way to say they are extremely...

...excited about what this may mean for the evolution and Improvement for Cancer Treatment as well as many other illnesses using Live Bio Therapeutics...
An area which @4dpharmaplc already own mass IP with 900+ patents in the area 💥📈🚀
This HUGELY VALIDATES that the Microbiome is the FUTURE for treatment💥🚀
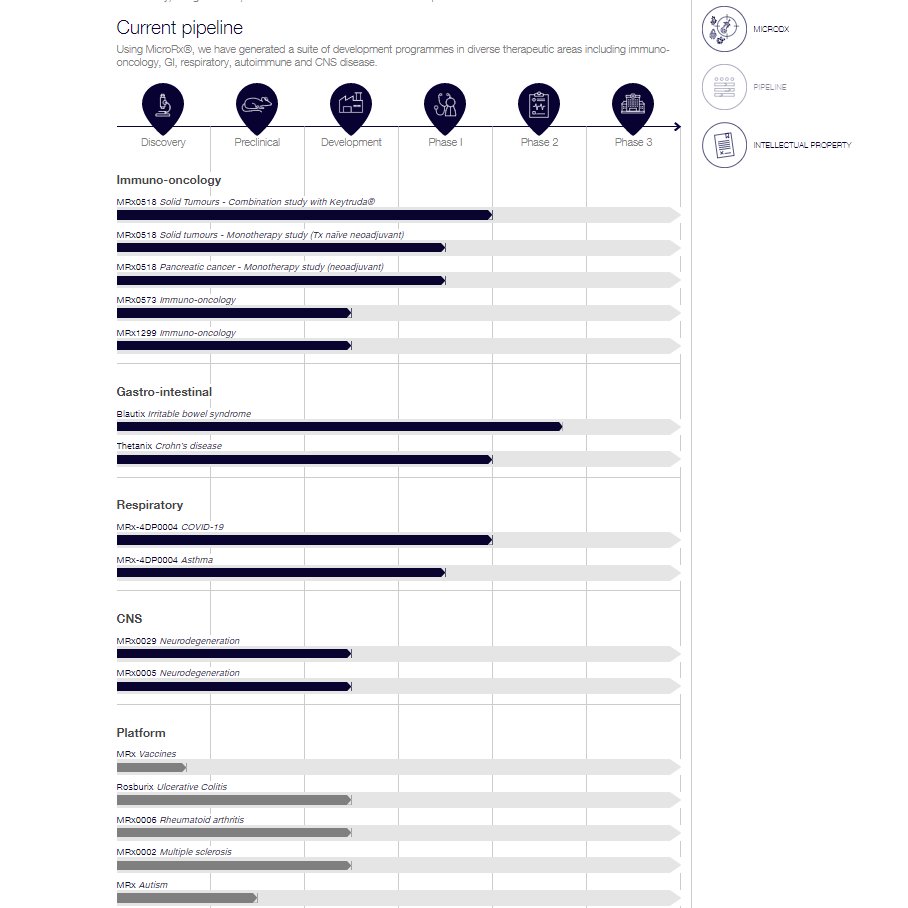
🤯🤯JUST LOOK AT THE SHEER SCALE OF WHAT WE ARE WORKING ON🤯🤯
WAIT TILL WE LIST ON NASDAQ📈
£40+ a share is my first target💥🔥
🚨CEO- “The responses that we are seeing are PHENOMENAL really”🚨
-Autumn➡️ Discussion with regulators (FDA) for Accelerated Approval
-2021➡️ Pivotal Trial for ACCELERATED Approval
Further discussions with Merck and potential other partners🔥