The PM claimed that they have validated these new 15min antigen tests – what is the @dhsc @phe validation process?
15 Aug ministers commissioned new process at PHE Porton Down in collaboration with Oxford University. Protocol here (updated 23 Oct).
gov.uk/government/pub…
15 Aug ministers commissioned new process at PHE Porton Down in collaboration with Oxford University. Protocol here (updated 23 Oct).
gov.uk/government/pub…
3 phases:
1 document review
2A Test 60 spiked saliva samples (n=15x4 dilutions)+71 -ve samples
2B Test against seasonal coronaviruses
3 Lab study of 1000 negatives and 200 positives sourced by Oxford University Hospitals. -ves fresh (<48hr) saliva samples +ves frozen
1 document review
2A Test 60 spiked saliva samples (n=15x4 dilutions)+71 -ve samples
2B Test against seasonal coronaviruses
3 Lab study of 1000 negatives and 200 positives sourced by Oxford University Hospitals. -ves fresh (<48hr) saliva samples +ves frozen
“Phase 3 findings will be reported to the Oversight Group, with DHSC and ministers using this information and any recommendations to inform potential purchasing decisions.”
Little detail is public – full results below - criteria for “pass” and “fail” for Phase 3 not stated
Little detail is public – full results below - criteria for “pass” and “fail” for Phase 3 not stated

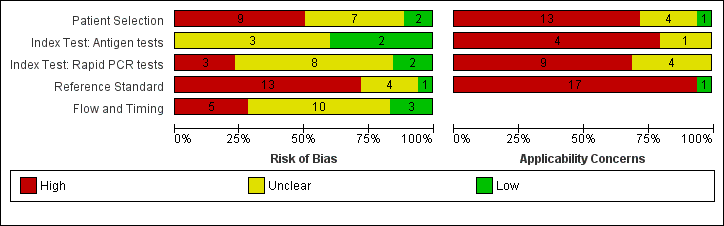
Its rather hard to find any criterion on the STARD checklist that this meets. This level of reporting is nowhere near the hopes of @RoyalStatSoc call for transparency. @DHSCgovuk @PHE_uk can you release full reports please as you have done before?
This is a lab based study on pre-selected samples, thus will only provide estimates akin to analytical accuracy, not estimates of diagnostic accuracy in the settings where they will be applied.
Boris described these as “tests of whether you are infectious” last night. There is no mention of infectiousness in the protocol – they are assessed as tests of infection not infectiousness.
• • •
Missing some Tweet in this thread? You can try to
force a refresh