A short thread on vaccine efficacy versus vaccine effectiveness, covering:
- Idealized vs. real-world effects
- Direct vs. indirect effects
- Individual-level vs. population-level effects
- Phase III vs. Phase IV trials
👇👇👇
- Idealized vs. real-world effects
- Direct vs. indirect effects
- Individual-level vs. population-level effects
- Phase III vs. Phase IV trials
👇👇👇
https://twitter.com/ZoeMcLaren/status/1330596361109114882
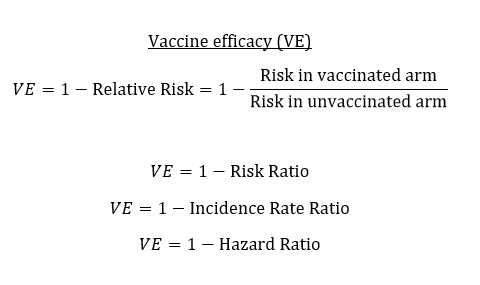
Phase 3 trials are conducted under idealized conditions. Everyone receives all doses on time, that have been properly stored, etc. The primary analysis is restricted, like to people without antibodies at baseline. We call the resulting estimate "vaccine efficacy." 2/8
Think "vaccine efficacy" as our best guess at the biological protection of the vaccine. When we talk about "vaccine effectiveness," this can refer to a few different things. One is "real-world effectiveness." If conditions are less than ideal, how well does the vaccine work? 3/8
Another concept of effectiveness is the "effectiveness of a vaccination program." Efficacy is an individual-level measure of protection, which we also call a "direct" effect. But vaccines can also have "indirect" protection, by preventing infection or reducing infectiousness. 4/8
We can measure the impact of a vaccination program at a population-level, instead of at an individual level. If you have an area where 50% of the population is vaccinated, versus an area where no one is vaccinated, how much better off is the partially vaccinated area? 5/8
This population-level effect is known as "overall vaccine effectiveness," and it captures both the direct protection and the indirect protection. In this way, it depends upon the vaccine coverage. It may also be highly location-specific. 6/8
A common post-licensure (Phase IV) design is a cluster randomized trial where the outcome is disease across both vaccinated and unvaccinated community members. These are known as "effectiveness trials."
A typhoid vaccine example is provided: 7/8
nejm.org/doi/full/10.10…
A typhoid vaccine example is provided: 7/8
nejm.org/doi/full/10.10…
Thus, while vaccine efficacy is narrowly defined, effectiveness is a broader term that captures both how well the vaccine works under real-world conditions, and, for overall effects, its population-level impact. 8/END
bmcmedicine.biomedcentral.com/articles/10.11…
bmcmedicine.biomedcentral.com/articles/10.11…
• • •
Missing some Tweet in this thread? You can try to
force a refresh