I have the rest of the week off, so when I take a break from #amwriting, expect a bunch of these.
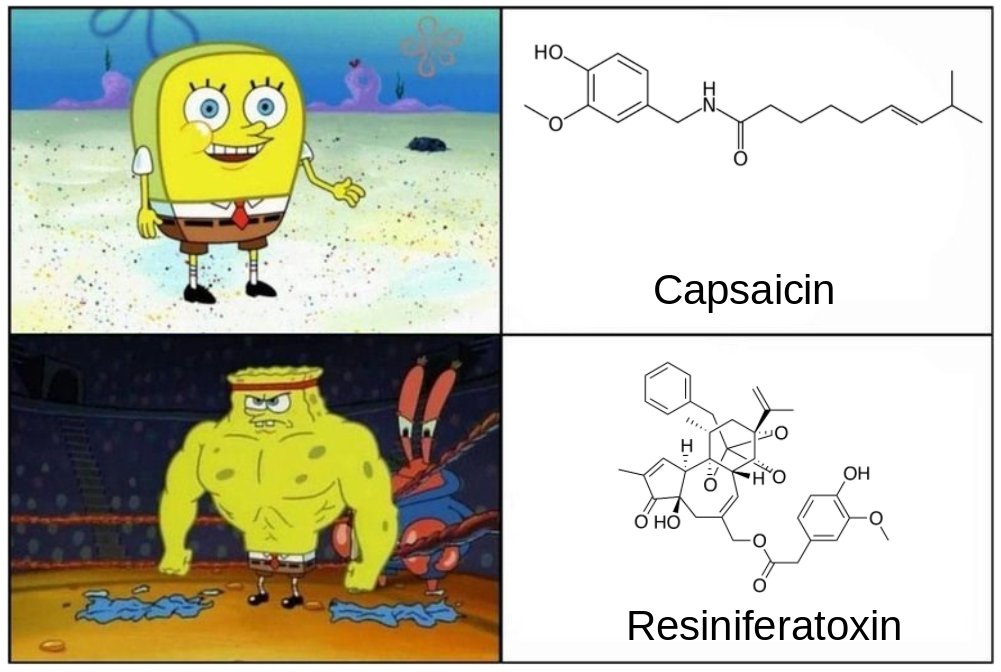
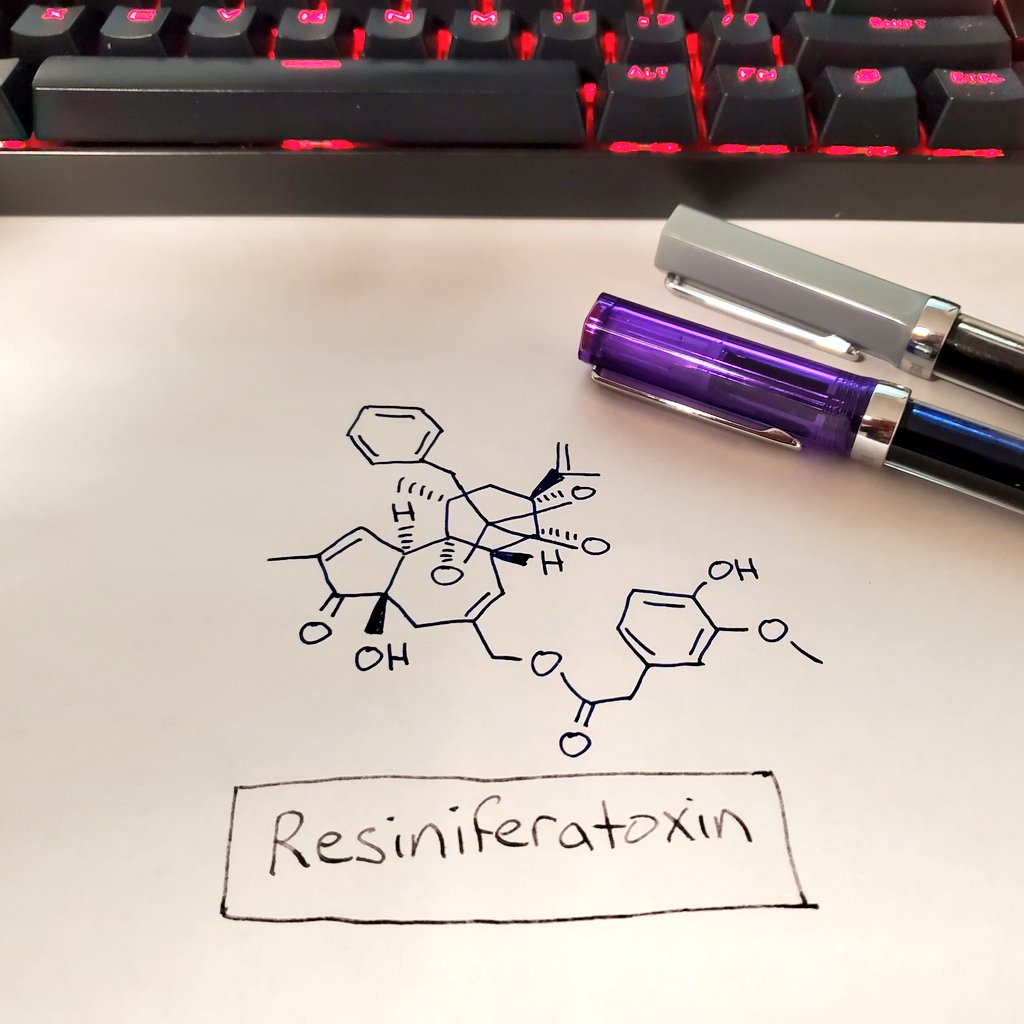
You've heard of capsaicin, right? The the "hot" chemical in chili peppers? Today I present you with RESINIFERATOXIN. It's 🔥🔥🔥!
You've heard of capsaicin, right? The the "hot" chemical in chili peppers? Today I present you with RESINIFERATOXIN. It's 🔥🔥🔥!
The resin spurge plant, Euphorbia resinifera is a cactus-y type thing found in north Africa. If you cut it open, it exudes a milky latex that contains resiniferatoxin. But watch out! [Pic by James Steakley (CC BY-SA 3.0)] 

Resiniferatoxin is about 1000-times "hotter" than capsaicin. It's likely the hottest, most painful toxin on the planet, so I advise not putting it in your chili or making a hot sauce out of it.
It works the same way as capsaicin. It's an agonist of the transient vanilloid receptor 1. To be a little less geeky, it activates TRPV1. It causes this ion-channel to stay open, depolarizing neurons as if they are transmitting a signal that screams PAIN. It does this very well.
As little as one-millionth of a gram, a nanogram (1/1,000,000), is painful. The funny thing though, is after a while all that stimulation results in desensitization, meaning you feel no pain. It becomes an analgesic because all the nerve endings are overloaded and fried.
So in that regard, if you can wait out the pain for the analgesic effect to kick in, it could be beneficial. We see that with topical capsaicin creams, so it's no surprise we have clinical trials with resiniferatoxin, like one injecting it into the knee for osteoarthritis.
One group even looked at it as a drug for treating premature ejaculation in dudes. They quite simply soaked the penis in a solution of resiniferatoxin. It worked too...because you can't ejaculate if your DICK IS ON FIRE.
Anyways, that's resiniferatoxin, the chemical that is about 1000-times hotter than capsaicin. Now back to #amwriting. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh