Happy Thanksgiving to those of you in the US! Happy Thursday to the rest of y'all.
I told you these were coming during my #amwriting breaks! In the spirit of overeating and edible things, here's the toxic tale of COPRINE.
I told you these were coming during my #amwriting breaks! In the spirit of overeating and edible things, here's the toxic tale of COPRINE.

The inkcap mushrooms, or "inky caps", are edible mushrooms with a mild flavor. I know, you're here for the poisonous mushrooms, not the edible ones. It is edible, but not when you consume them with alcohol! Weird, I know, but I'll explain. [pic by Nick Saltmarsh (CC BY-2.0)] 

The ink caps contain a chemical called COPRINE. It's not too special, though the cyclopropyl group (the triangle part) is always fun to see in natural products. The problem with COPRINE is when it is metabolized. When ingested, the body breaks it down to AMINOCYCLOPROPANOL. 

What the hell is AMINOCYCLOPROPANOL you ask? It's an inhibitor - stops the function of - aldehyde dehydrogenase. If you drink alcohol, you're very lucky to have these enzymes.
When you drink alcohol, your body wants to get rid of it. It does this by oxidizing ethanol to acetaldehyde with the enzyme alcohol dehydrogenase. Our bodies haaaates acetaldehyde, and further oxidizes it to acetic acid with aldehyde dehydrogenase. Ethanol crisis averted! 
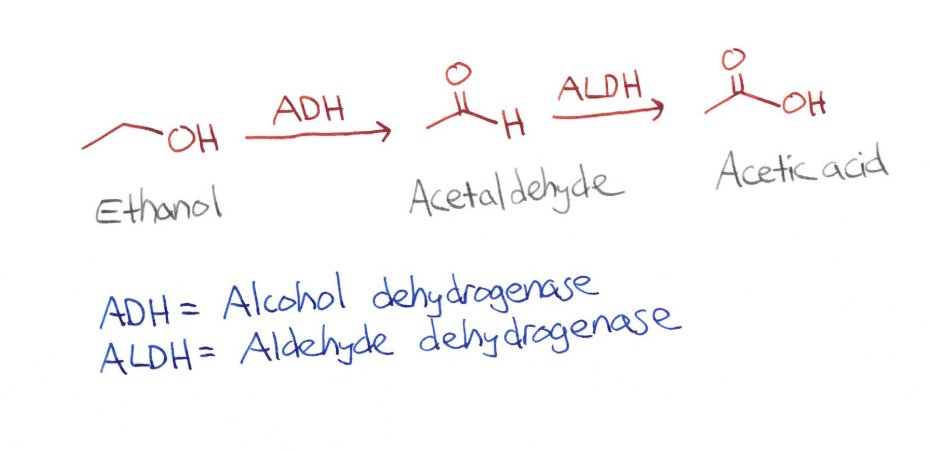
So I said our body hates acetaldehyde. It does. We do. Acetaldehyde is super toxic to the body. But it also makes us sick. It makes us nauseous and flushed - that "I'm never drinking again" feeling. So we need aldehyde dehydrogenase to get rid of it for us.
Since we already established AMINOCYCLOPROPANOL is an inhibitor of aldehyde dehydrogenase, it stops the conversion of acetaldehyde to acetic acid, building up acetaldehyde in the body, making you feel super sick and nauseous. 

What else works this way, as an inhibitor of aldehyde dehydrogenase? Disulfiram, also known as Antabuse, which is used as a negative reinforcement to drinking alcohol. So you take it daily, and if you consume alcohol, you'll get violently sick and feel like ass.
I love COPRINE because it's not a poison in and of itself, just in combination with alcohol. It's sneaky like that. It's an ED doctor's nightmare.
People come into the ED nauseous and sick and the doctor sees they ate wild mushrooms. Bingo! But not everyone that ate them got sick, and not the kids, who are usually the most susceptible to poisonings. AGHHH! Fortunately, I don't know of any fatalities, you just feel like ass.
So that's the story of COPRINE and the dangers of eating inky cap mushrooms with alcohol. A little bit of chemistry, biology, biochemistry, toxicology, and medicine all rolled into one toxic tale. Enjoy your day everyone! 

• • •
Missing some Tweet in this thread? You can try to
force a refresh