
NEW—First full results from interim analysis confirm that the Oxford #COVID19 vaccine is safe and efficacious against symptomatic COVID-19 disease: hubs.li/H0CcSs60 (1/6)
The vaccine protects against symptomatic disease in 70% of cases – with vaccine efficacy of 62% for those given two full doses, and of 90% in those given a half then a full dose. (2/6) 
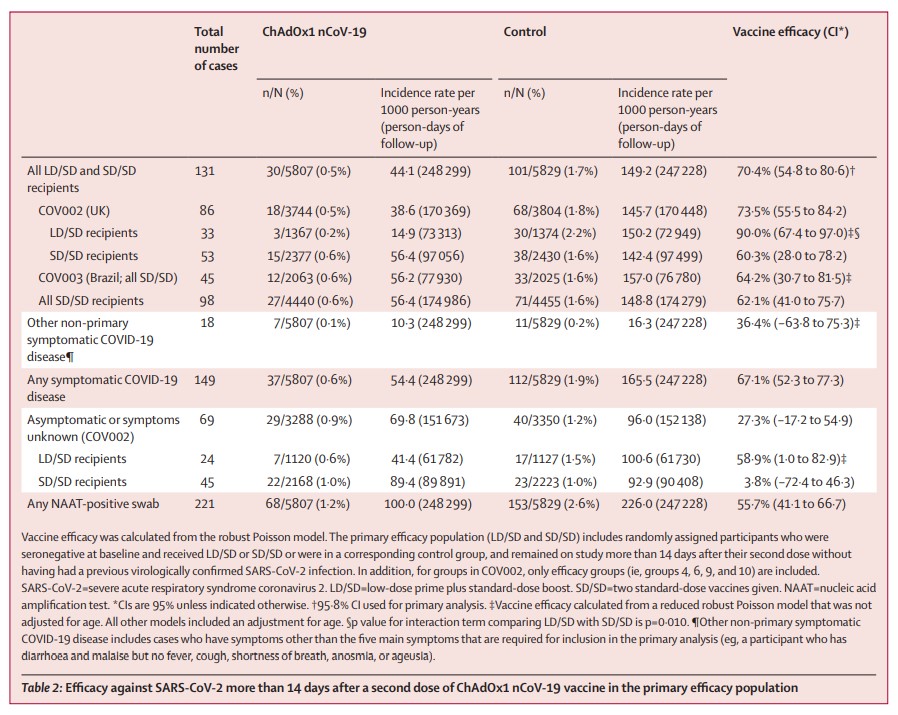
The first clinical efficacy results of the vaccine are based on a pre-specified pooled analysis of phase 3 trials in UK and Brazil (11,636 people), alongside safety data from 23,745 participants in 4 trials in the UK, Brazil and South Africa. (3/6) 
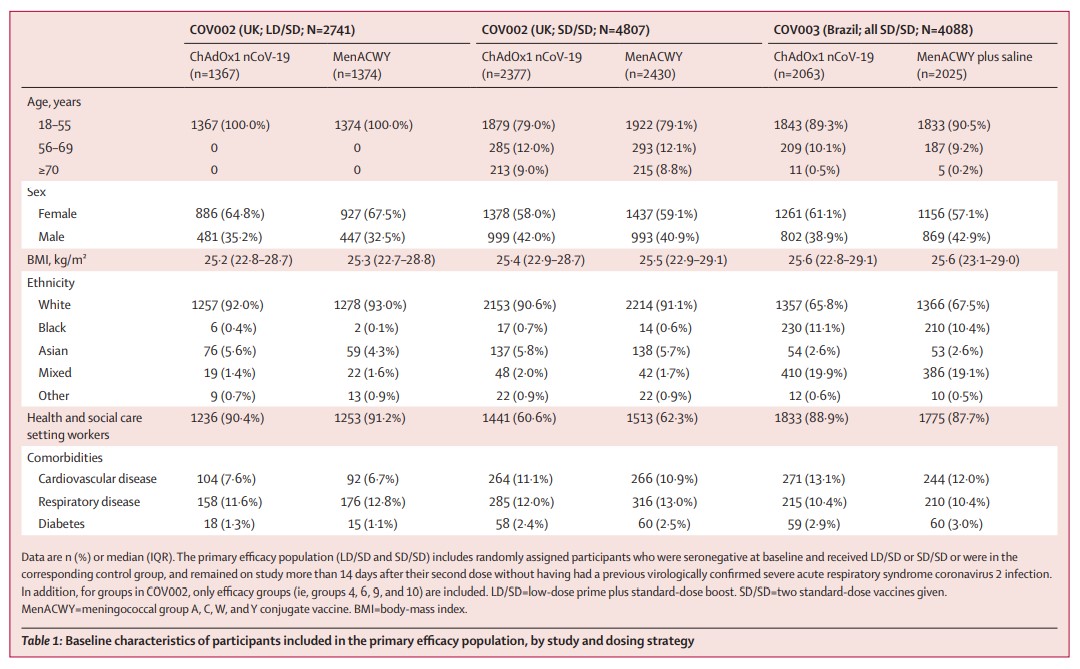
Only three out of 23,745 participants over 3.4 months experienced serious adverse events: 1 in vaccine group, 1 in control group & 1 remains masked to group allocation. All participants have recovered, or are recovering, and remain in the trial. (4/6)
Secondary outcomes suggest the vaccine may also protect against asymptomatic infection – vaccine efficacy of 4% for those given two full doses, and of 59% in those given a half then a full dose. However, findings need to be confirmed. (5/6)
As more data becomes available, authors will investigate differences in subgroups such as older adults, various ethnicities, doses, timing of booster vaccines, and will determine which immune responses equate to protection from infection or disease. (6/6)
• • •
Missing some Tweet in this thread? You can try to
force a refresh

















