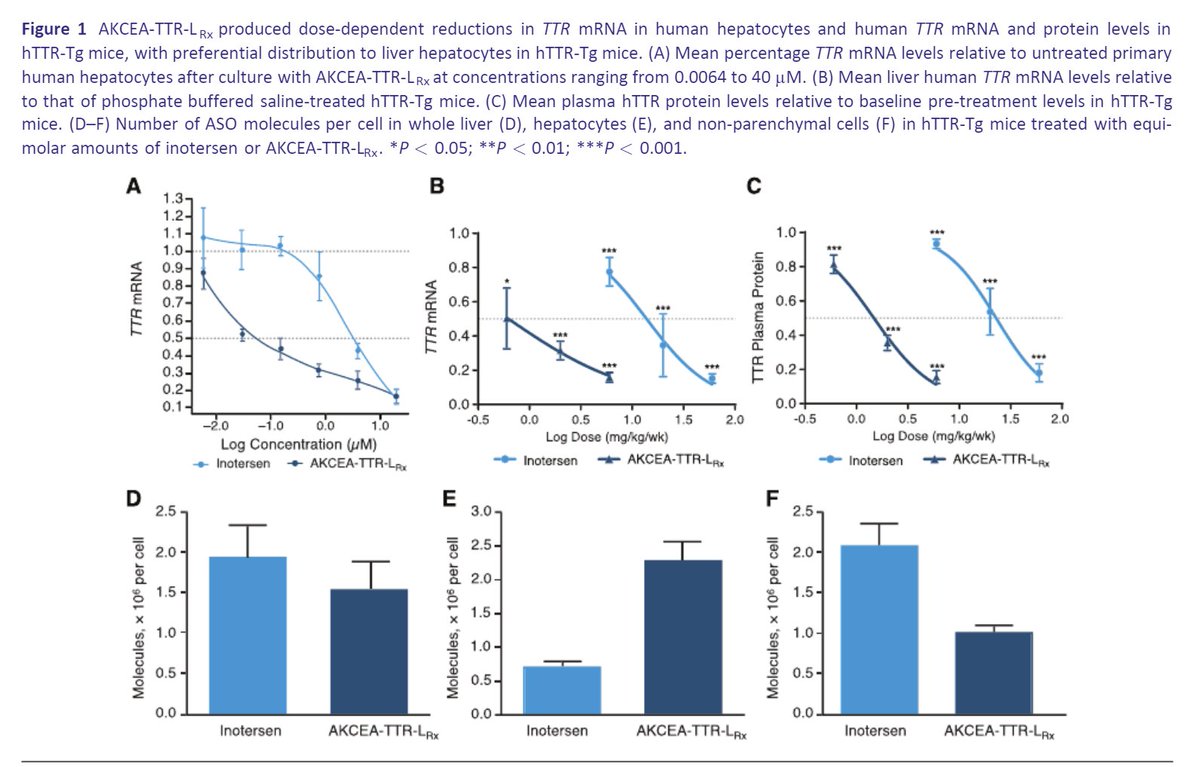
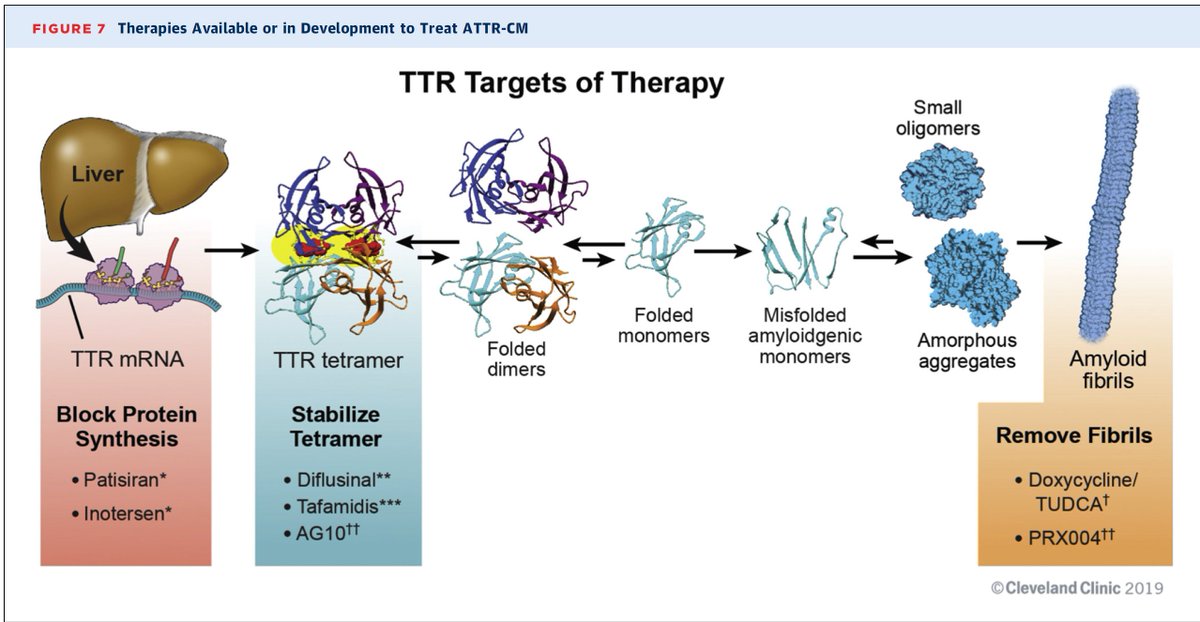
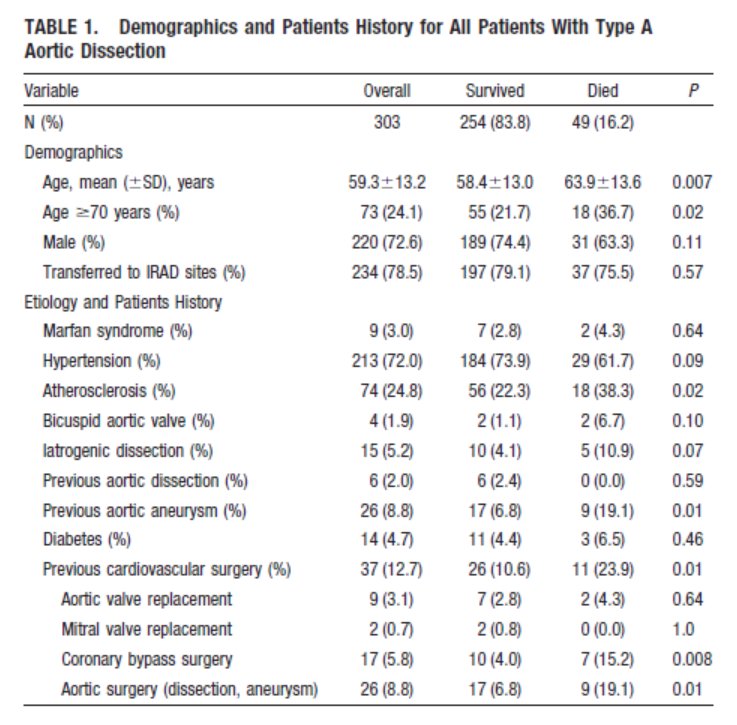
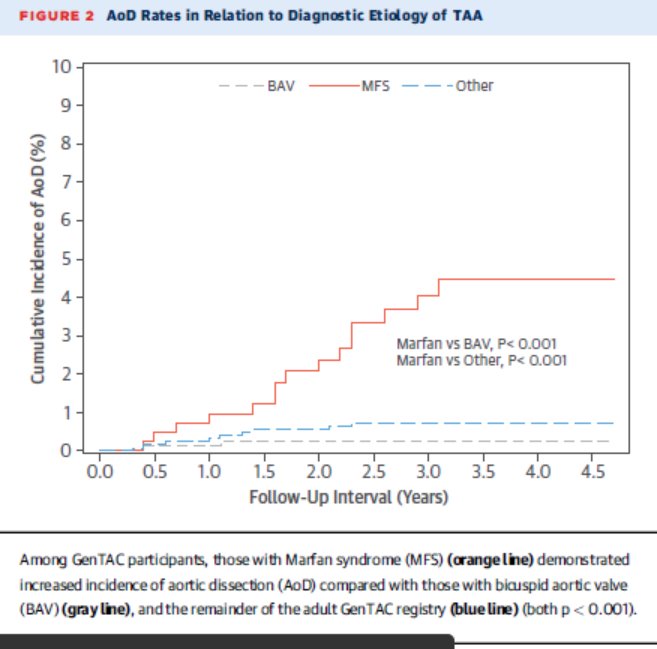
Preclinical and phase I data for $AKCA-TTR-LRx (second generation inotersen, ASO conjugated with GalNAc), currently being tested in CARDIO-TTRansform, the largest #ATTR #Amyloidosis trial to date. Tafamidis allowed without restrictions.
onlinelibrary.wiley.com/doi/epdf/10.10…
#cardiotwitter

onlinelibrary.wiley.com/doi/epdf/10.10…
#cardiotwitter


more about the trial here
https://twitter.com/MasriAhmadMD/status/1274455645261934593?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh