The #ACIP meeting is starting. It looks like they have revised their 1b recommendation (still to be voted on) to be those 75+ and frontline essential workers.
cdc.gov/vaccines/acip/…
cdc.gov/vaccines/acip/…
1c) rec will be those aged 65–74 years; those aged 16–64 years with high-risk medical conditions; and other essential workers.
Teachers are in ACIP's definition of frontline essential workers.
Not just teachers - also support staff, daycare staff.
Other frontline essential workers: first Responders, Food & Agriculture, Manufacturing, Corrections workers, U.S. Postal service workers, Public transit workers, Grocery store workers
Other frontline essential workers: first Responders, Food & Agriculture, Manufacturing, Corrections workers, U.S. Postal service workers, Public transit workers, Grocery store workers
Here is what ACIP is trying to balance. This is 100% due to the fact that vaccines have to be rationed because supply is limited. We are seeing the necessary rationing of life saving interventions in action, b/c we don't have enough vaccine. We should thank ACIP for this work. 

Decisions about moving into the next phase should be based on supply (and demand).
To aid state and local jurisdictions in further sub-prioritization, ACIP recommends:
– Workers in locations where high rates of transmission and/or outbreaks; workers at increased risk for severe illness; workers w/no history of documented acute infection in prior 90 days.
– Workers in locations where high rates of transmission and/or outbreaks; workers at increased risk for severe illness; workers w/no history of documented acute infection in prior 90 days.
A really important statement from ACIP just now: Based on local epidemiology and implementation considerations,
jurisdictions may choose to vaccinate frontline essential workers AND those living in congregate settings (e.g., prisons, jails, homeless shelters) at the same time.
jurisdictions may choose to vaccinate frontline essential workers AND those living in congregate settings (e.g., prisons, jails, homeless shelters) at the same time.
A question just now about how many racial/ethnic minorities are in the frontline vs other essential worker group. Answer: more likely to be in the "other" essential worker group.
One of ACIP's considerations (for suggesting priorities in this initial phase) is who is more likely to be able to stay at home vs must work and does not have the ability to telework. This is where the balance between those 65-74 and frontline worker tips a bit.
Funding keeps coming up since much needed funding for vaccine distribution is being held "hostage" to the larger stimulus negotiation (vaccine $$ is not at issue). So far, state and local jurisdictions have received $340 million. It's estimated they need $6-$8 billion.
A number of ACIP members have talked about how wrenching these decisions are (and reiterated that prioritization is only being done b/c supply is limited).
To define high risk conditions, ACIP is relying on CDC's ongoing research on who is at risk for severe COVID-19 disease.
cdc.gov/coronavirus/20…
cdc.gov/coronavirus/20…
Question asked about whether medical students are included in the definition of health care workers. Answer - Yes.
Several people have talked about the importance of local flexibility in prioritization but also how this will lead to lots of variation. We have already found that in our review of state priorities, particularly for the rest of Phase 1:
kff.org/policy-watch/h…
kff.org/policy-watch/h…
This is of course expected. But it also will mean that access will vary depending on where one lives (as it always does with health care in the U.S.).
Point raised by Dr. Lee now that equity needs to be monitored and assessed as vaccine roll-out happens. "We cannot abandon equity because it's hard to measure"
Equity across the nation can really only be measured and assured by the federal government.
Sorry - putting a few tweets that got away into my thread:
https://twitter.com/jenkatesdc/status/1340720557411721216?s=20
One view just expressed (missed who) that we will get through 1b fast so need to be prepared to help reach those in 1c. Not sure we will get through that fast, actually.
Some have raised the idea of putting those ages 64-75 yr olds in 1b. Answers include:
*easier to reach the targeted 75+ first
*supply projections are just projections and not a guarantee
*easier to reach the targeted 75+ first
*supply projections are just projections and not a guarantee
Some ACIP members are suggesting pushing the discussion about expanding the 1b age group to another meeting.
Here is what #ACIP is voting on. 

CDC says it will clarify some of the issues that have been raised in the discussion, in its clinical considerations document that will accompany the final recommendation.
Some ACIP members have proposed just voting on 1b today and waiting on 1c for more clarification. Most seem to want to go forward with the original recommendation on both.
The ACIP member who requested potentially splitting to vote just on 1b today withdraws the motion. Is fine with the vote as proposed. But wants more clarification and discussion on 1c afterwards.
Public comment period has just started.
An observation (certainly not an original one): while we are all worried about vaccine hesitancy and searching for ways to help educate about the importance of the COVID-19 vaccine, there are a lot of people who really, really want this vaccine. Most, in fact.
From our new COVID-19 Vaccine Monitor Project, we found that 71% of the public says they will definitely or probably get vaccinated.
kff.org/coronavirus-co…
kff.org/coronavirus-co…
Public comment at this, and prior, ACIP meetings has mostly been people and groups pleading to get earlier access to the vaccine.
The Chair of the ACIP is now making a statement about some of the misinformation and critique of ACIP, impugning the work of ACIP, noting that the ACIP has struggled "painfully to deal with distribution of a limited resourced vaccine".
He continues: "The statements being made through various outlets undermine the trust and....work of our committee". He says that ACIP has never focused on targeting a specific racial or ethnic group in its deliberations.
ACIP is now reviewing the list of clinical conditions with evidence of posing a risk for severe COVID19 disease.
https://twitter.com/jenkatesdc/status/1340711581617950720?s=20
Vote is about to start.
Vote is 13 to 1 to support the recommendation:
Phase 1b: persons aged ≥75 years and frontline essential workers
Phase 1c: persons aged 65–74 years, persons aged 16–64 years with high-risk medical conditions, and other essential workers
Phase 1b: persons aged ≥75 years and frontline essential workers
Phase 1c: persons aged 65–74 years, persons aged 16–64 years with high-risk medical conditions, and other essential workers
The @cdc director still needs to accept this recommendation. It is advisory. Also, states do not have to follow this.
ACIP voting members are now making comments about their vote (they are always given time to do so). To a person, they are talking about how difficult it has been to make these decisions.
That's a wrap. Ending with one more piece of information.
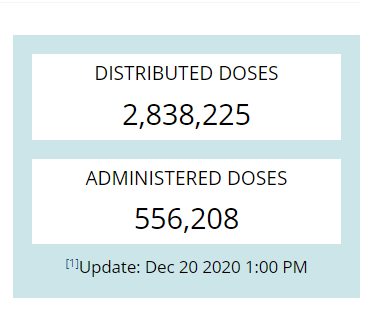
2.8+ million doses have been distributed
556K doses administered.
cdc.gov/coronavirus/20…
2.8+ million doses have been distributed
556K doses administered.
cdc.gov/coronavirus/20…
• • •
Missing some Tweet in this thread? You can try to
force a refresh