@EpiEllie @mlipsitch @vivek_murthy Thanks @EpiEllie for the ?
A number of issues:
1) Regulatory - FDA must remove unnecessary regulatory requirements
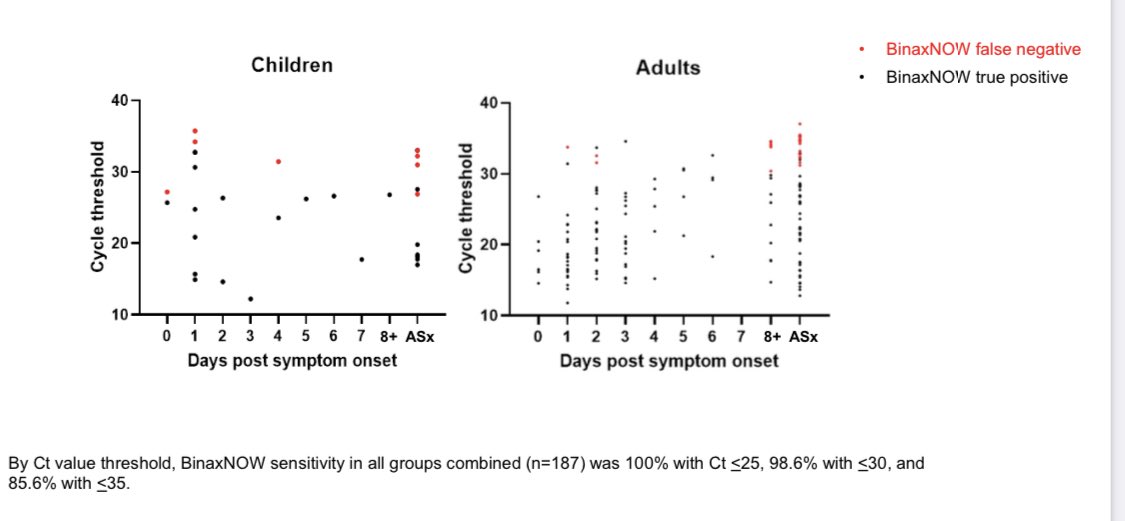
Most crucial: ~90% sensitivity against PCR in ASYmptomatics w/out allowing a Ct cutoff
By time asymptomatics are found w PCR, Ct is >30 & post-infectious
1/x
A number of issues:
1) Regulatory - FDA must remove unnecessary regulatory requirements
Most crucial: ~90% sensitivity against PCR in ASYmptomatics w/out allowing a Ct cutoff
By time asymptomatics are found w PCR, Ct is >30 & post-infectious
1/x
@EpiEllie @mlipsitch @vivek_murthy By requiring demonstrating sensitivity specifically in asymptomatics, FDA is confusing EUA application with showing physiology of the virus.
Many studies show asymptomatics have virus.
The tests care about virus, not symptoms. FDA is confusing this point.
2/x
Many studies show asymptomatics have virus.
The tests care about virus, not symptoms. FDA is confusing this point.
2/x
@EpiEllie @mlipsitch @vivek_murthy Reason FDA confuses this is b/c they can ONLY see tests as clinical medical diagnostic devices
Not as public health screening tools
This means their metrics and what they are looking for are not congruent with their intended use in this case. Which is a major problem.
3/x
Not as public health screening tools
This means their metrics and what they are looking for are not congruent with their intended use in this case. Which is a major problem.
3/x
@EpiEllie @mlipsitch @vivek_murthy Another problem is need for prescription
We now have a completely confusing landscape where some #COVID19 tests are prescription and some are not.
This makes no sense. Particularly since one of the most technically challenging to use is the one authorized at-home OTC test.
4/x
We now have a completely confusing landscape where some #COVID19 tests are prescription and some are not.
This makes no sense. Particularly since one of the most technically challenging to use is the one authorized at-home OTC test.
4/x
@EpiEllie @mlipsitch @vivek_murthy Other major issues are need to allocate the financial resources both to help the companies scale capacity and to produce the tests.
If USG doesn't give grants now to help them scale, then they will charge much more $/test later to cover costs. More than initial build out.
5/x
If USG doesn't give grants now to help them scale, then they will charge much more $/test later to cover costs. More than initial build out.
5/x
@EpiEllie @mlipsitch @vivek_murthy Many states governors want to put these tests into use.
But simply cannot afford it. It's pennies for the federal government, but $100M is a lot for any state.
So this needs to be an effort of the federal government first and foremost. Even if championed by the states.
6/x
But simply cannot afford it. It's pennies for the federal government, but $100M is a lot for any state.
So this needs to be an effort of the federal government first and foremost. Even if championed by the states.
6/x
@EpiEllie @mlipsitch @vivek_murthy Funding piece is crucial, which is why I've spent a lot of time lobbying congress to get it into the bill.
Unfortunately I failed this time around.
They vaguely worded it and suggested the states could each come together to do this. That's BS, must be central scaling
7/x
Unfortunately I failed this time around.
They vaguely worded it and suggested the states could each come together to do this. That's BS, must be central scaling
7/x
@EpiEllie @mlipsitch @vivek_murthy Also many physicians/scientists just can't see obvious problems with slow and infrequent PCR vs rapid frequent testing.
Which we have demonstrated well is not a problem.
Some of those qualms are here:
Which we have demonstrated well is not a problem.
Some of those qualms are here:
https://twitter.com/michaelmina_lab/status/1340939361710424064?s=20
• • •
Missing some Tweet in this thread? You can try to
force a refresh