IMPORTANT:
Particularly for UK
There’s been a LOT of concern about UK rapid antigen test program.
But here, the very tweet supposed to be showing it is failing, it shows it’s working well!!
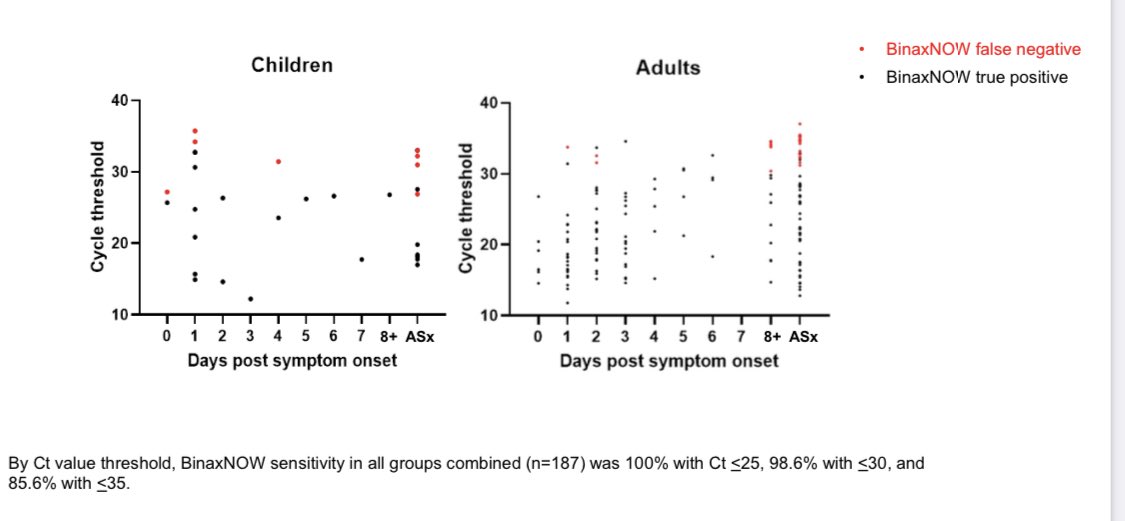
The only missed samples were all very LOW PCR RNA - EXACTLY as expected.
1/x
Particularly for UK
There’s been a LOT of concern about UK rapid antigen test program.
But here, the very tweet supposed to be showing it is failing, it shows it’s working well!!
The only missed samples were all very LOW PCR RNA - EXACTLY as expected.
1/x
https://twitter.com/deeksj/status/1340975399128457216
The rapid antigen tests caught all of the high viral load cases that were likely contagious and missed the low RNA level cases who were most likely beyond their contagious period.
This suggests the testing was simply too infrequent to catch the important cases.
2/x
This suggests the testing was simply too infrequent to catch the important cases.
2/x
At what point are we going to stop making the claim that these tests are too insensitive?
They are doing the job exactly as anticipated. Public health is not about diagnosing and isolating old COVID cases. It’s about identifying current contagious people.
3/3
They are doing the job exactly as anticipated. Public health is not about diagnosing and isolating old COVID cases. It’s about identifying current contagious people.
3/3
To clarify a central issue with Birmingham Rapid Antigen Test study:
The study results show that for:
Ct < 30, Sensitivity = 5%
Ct < 29, Sensitivity = 100%
Any time the sensitivity jumps from 5% to 100% in a SINGLE Ct value, you know there is a major problem with the study.
The study results show that for:
Ct < 30, Sensitivity = 5%
Ct < 29, Sensitivity = 100%
Any time the sensitivity jumps from 5% to 100% in a SINGLE Ct value, you know there is a major problem with the study.
Also, in that tweet thread it mentions half the positives in a different study were false positives. Not mentioned was this was due entirely to low prevalence.
What the thread should have also mentioned in same breath was that specificity was >99.9%!! Very high specifity!
What the thread should have also mentioned in same breath was that specificity was >99.9%!! Very high specifity!
• • •
Missing some Tweet in this thread? You can try to
force a refresh