Since the UK just approved the AstraZeneca/University of Oxford COVID-19 vaccine today, I wanted to summarize the results of the interim analysis of their Phase 3 trials published in Lancet on December 8 with some slides:
thelancet.com/journals/lance…
thelancet.com/journals/lance…
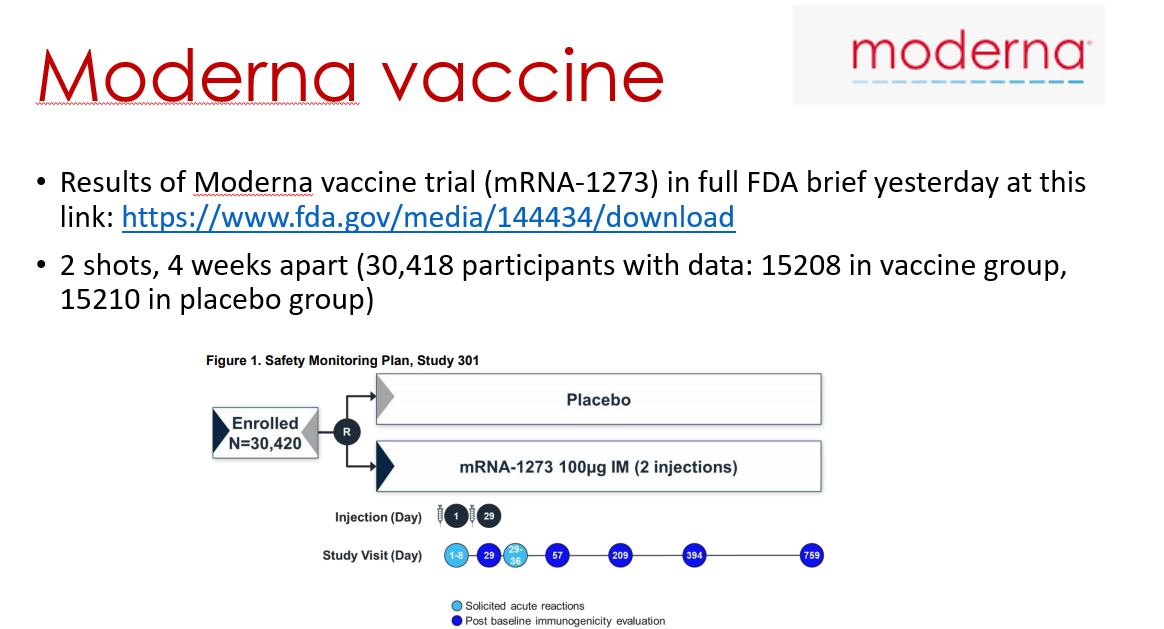
So, good about this vaccine (cheaper, storable in regular fridge for 6 months) but some strange and inconsistent things too (more efficacy with half, then full dose, and that done by accident). Forgot to say on slide that this is two doses given 4-12 weeks apart (aimed for 4)
Also, here is the New York Times article about the vaccine approval today in Britain with a nice infographic about how it works:
nytimes.com/2020/12/30/wor…
nytimes.com/2020/12/30/wor…
again, name of the game is speed which is why UK approved this - getting more people vaccinated (even one dose) with something that works even not perfectly will slow down the virus/cases/hospitalizations
• • •
Missing some Tweet in this thread? You can try to
force a refresh