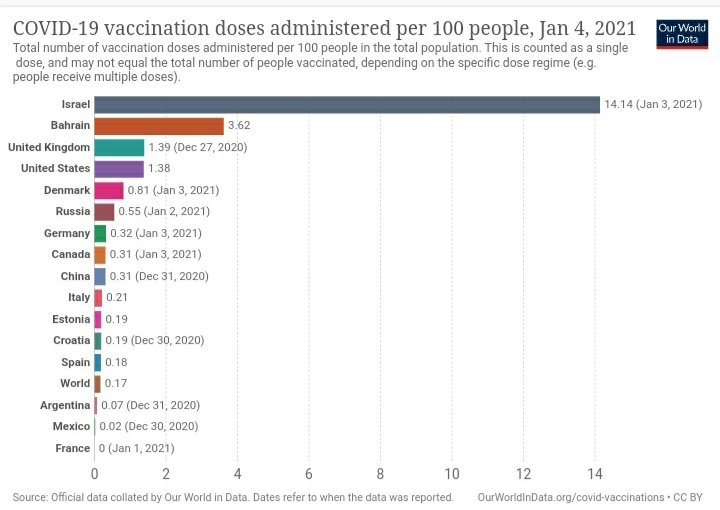
The expert committee of CDSCO met on 30/12, 01/01 and 02/01 to deliberate on accelerated approval of vaccines. Recommendations made on each day have been officially published.
📣 In this thread, we take a look at the timeline of events that lead to the approval. Must read.
1/n
📣 In this thread, we take a look at the timeline of events that lead to the approval. Must read.
1/n
30th December:
#Covaxin: Bharat Biotech presents status of Phase 3 trials and updated safety data.
📣 Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.
2/n
#Covaxin: Bharat Biotech presents status of Phase 3 trials and updated safety data.
📣 Committee recommends that updated safety and EFFICACY data of Phase 3 trials have to be presented for further consideration.
2/n

30th December:
#Covishield: SII presents safety and efficacy data of Phase 2/3 trials from UK, Brazil, SA along with data from ongoing Indian trials. SII mentions that UK has provided EUA.
📣 Committee asks for complete details of UK approval to be submitted.
3/n
#Covishield: SII presents safety and efficacy data of Phase 2/3 trials from UK, Brazil, SA along with data from ongoing Indian trials. SII mentions that UK has provided EUA.
📣 Committee asks for complete details of UK approval to be submitted.
3/n
1st January:
#Covaxin: Bharat Biotech presents data from Phase 1,2 trials as well as ongoing Phase 3. Efficacy yet to be demonstrated.
📣 Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.
4/n
#Covaxin: Bharat Biotech presents data from Phase 1,2 trials as well as ongoing Phase 3. Efficacy yet to be demonstrated.
📣 Committee asks for Phase 3 recruitment to be expedited and present Phase 3 efficacy data for consideration for emergency use approval.
4/n

1st January:
#Covishield: SII presents details of UK approval.
📣 Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials.
Decides to recommend for restricted emergency use.
5/n

#Covishield: SII presents details of UK approval.
📣 Committee reviews data and notes that the safety/immunogenicity data of India trials is comparable to overseas trials.
Decides to recommend for restricted emergency use.
5/n


Conditions of #Covishield restricted approval:
✅ Approved for >= 18 years of age.
✅ 2 doses, 4 to 6 weeks apart.
✅ SII should submit details of of ongoing Phase 3 studies.
✅ Safety data to be submitted every 15 days for first 2 months.
✅ Submit risk management plan.
6/n
✅ Approved for >= 18 years of age.
✅ 2 doses, 4 to 6 weeks apart.
✅ SII should submit details of of ongoing Phase 3 studies.
✅ Safety data to be submitted every 15 days for first 2 months.
✅ Submit risk management plan.
6/n

2nd January:
#Covaxin: Bharat Biotech presents data. Requests for approval in light of new mutated strain.
📣 Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains.
Phase 3 to continue.
7/n
#Covaxin: Bharat Biotech presents data. Requests for approval in light of new mutated strain.
📣 Committee recommends for emergency use in clinical trial mode as a precaution, to have more vaccine options in light of mutant strains.
Phase 3 to continue.
7/n
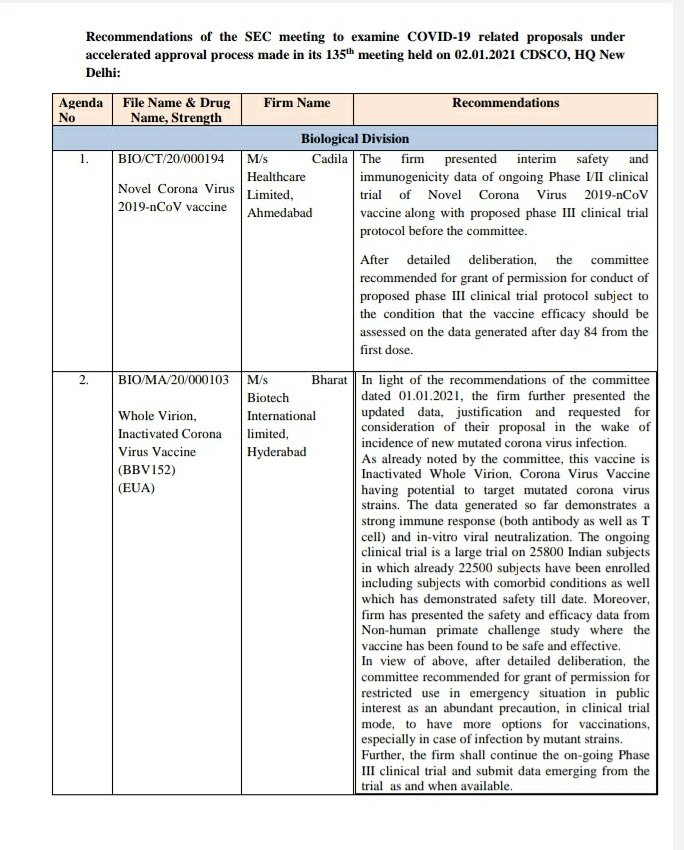
In summary:
✅ Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India.
✅ #Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations.
8/n
✅ Approval for Covishield provided on 01/01 after reviewing UK approval and trial data globally and in India.
✅ #Covaxin: In the meetings held on 30/12 and 01/01, SEC had asked for Phase 3 efficacy data to be presented for approval considerations.
8/n
In the meeting on 02/01, SEC decides to recommend Covaxin for emergency approval in clinical trial mode, without efficacy data from Ph 3 trials.
Reason given being to have more vaccine options in light of mutated strains. Clarification of clinical trial mode not provided.
9/n
Reason given being to have more vaccine options in light of mutated strains. Clarification of clinical trial mode not provided.
9/n
The detailed recommendation documents of SEC by date can be found here:
cdsco.gov.in/opencms/opencm…
n/n
cdsco.gov.in/opencms/opencm…
n/n
• • •
Missing some Tweet in this thread? You can try to
force a refresh