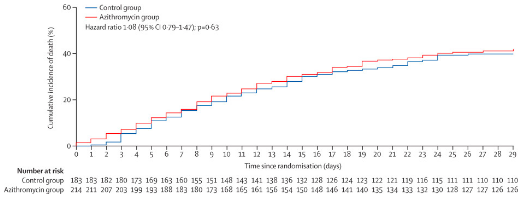
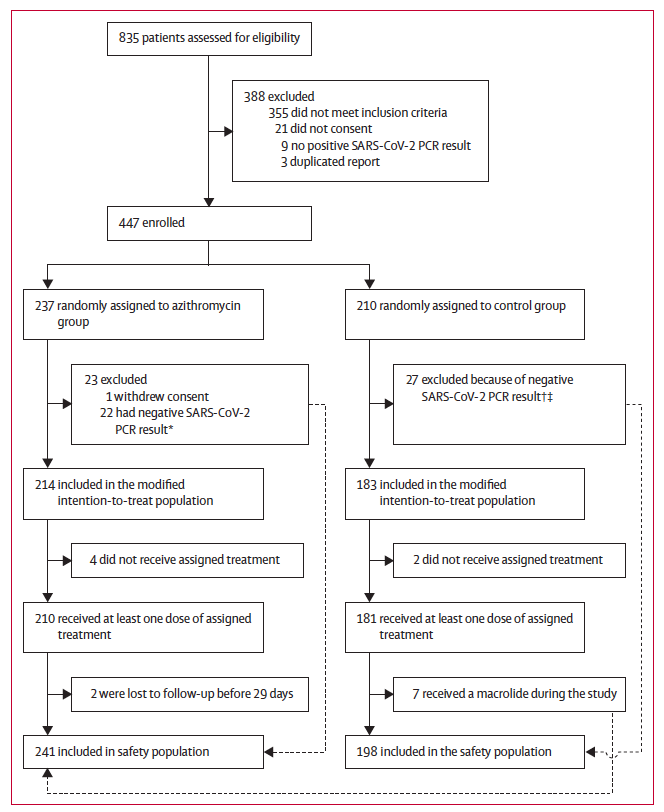
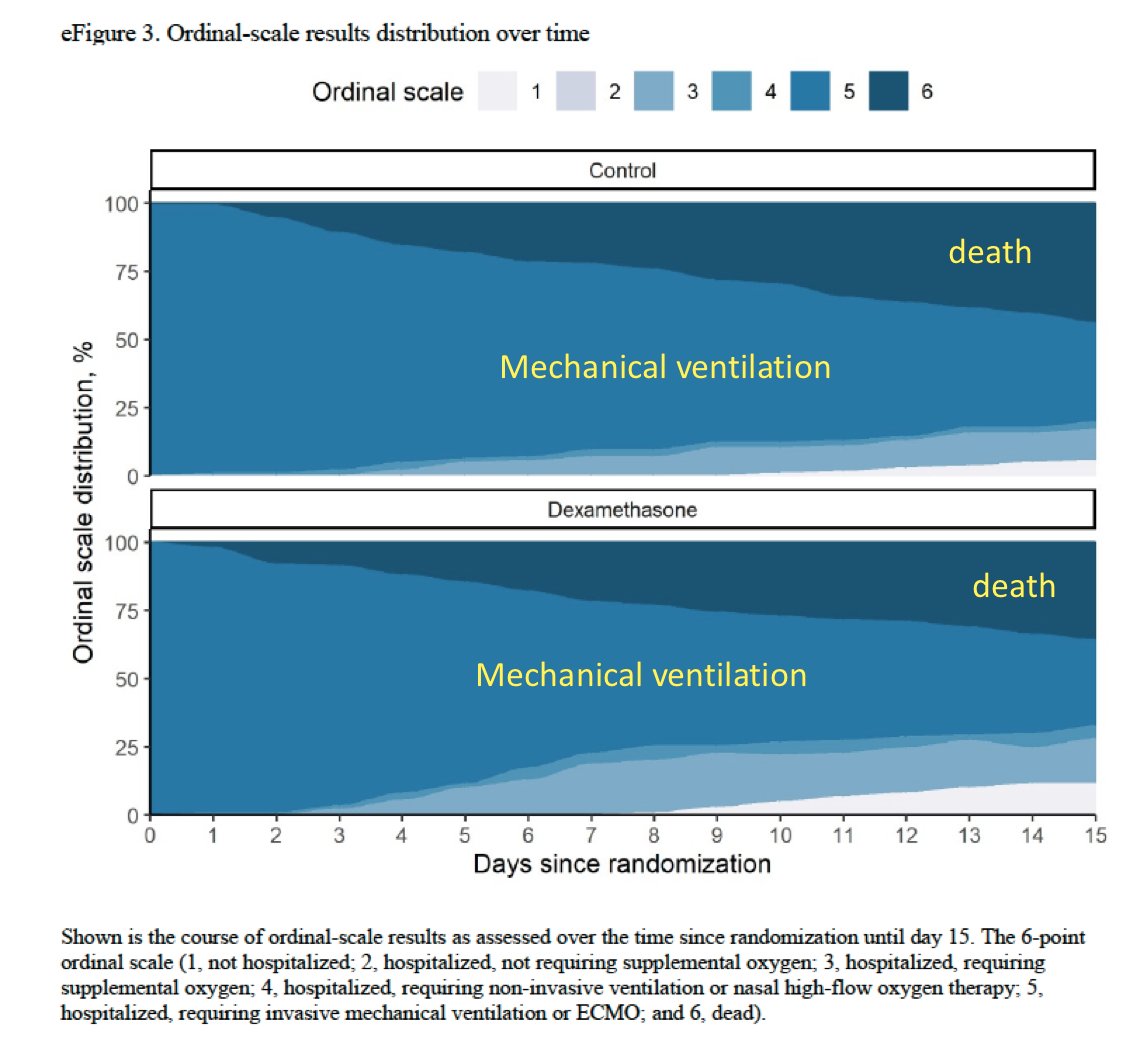
1/ Colleagues from Manaus reaching out in complete distress:
Covid19 patients that were *improving* are experiencing extreme anguish/panic while dying due to lack of Oxygen!
A system does not colapse out of the blue!
Avoidable catastrophe:
Covid19 patients that were *improving* are experiencing extreme anguish/panic while dying due to lack of Oxygen!
A system does not colapse out of the blue!
Avoidable catastrophe:

2/ NO BEHAVIORAL MEASURE to stop Covid19 was implemented in Manaus (nor anywhere else) in the last 4 weeks.
The population is left to its own luck (or lack of)
The President keeps disencouraging face masks, social distancing, and, BELIEVE ME, vaccination!
The population is left to its own luck (or lack of)
The President keeps disencouraging face masks, social distancing, and, BELIEVE ME, vaccination!
3/ If this was not enough, his proposal to address the Covid19 crisis in Brazil is early treatment with hydroxycholoquine, ivermectin, nitazoxanide 😱
Stop this madness!
@ministerpublico @STF_oficial @SenadoFederal @camaradeputados
@RodrigoMaia
Stop this madness!
@ministerpublico @STF_oficial @SenadoFederal @camaradeputados
@RodrigoMaia
Read this!!! 😡
Colleagues sedating *recovering* patients in order to treat their extreme emotional anguish while facing the lack of O2 and hypoxia!
Leiam!!! Irresponsáveis!
@SenadoFederal @camaradeputados @STF_oficial @ministerpublico
Colleagues sedating *recovering* patients in order to treat their extreme emotional anguish while facing the lack of O2 and hypoxia!
Leiam!!! Irresponsáveis!
@SenadoFederal @camaradeputados @STF_oficial @ministerpublico

• • •
Missing some Tweet in this thread? You can try to
force a refresh