#WCLC20 Results from phase I study of the TROP2 ADC datopotamab deruxtecan (Dato-DXd; DS-1062) in NSCLC by @AlexSpiraMDPhD #LCSM @IASLC @OncoAlert 

#WCLC20 This ADC includes an anti-TROP2 IgG1 and a topo-1 inhibitor payload. Phase 1 design shown here. Mostly non-squamous, included some #EGFR+, heavily pretreated including many with CNS metastases. Less intensity at higher 8mg dose, as one would expect. #LCSM 







#WCLC20 Safety shown here and manageable but I would say still notable, especially at 8mg (G3+ AEs 34%) including ILD noted in 8% (but 3 grade 5 cases in the 8mg/kg cohort). #LCSM 



#WCLC20 Exciting efficacy signal here (in a heavily pretreated population). RR 20% at 8mg dose (8% at 6mg/kg) but mPFS longest in 6mg cohort (8.2m). Spider plots show some very deep and durable responses. #LCSM 
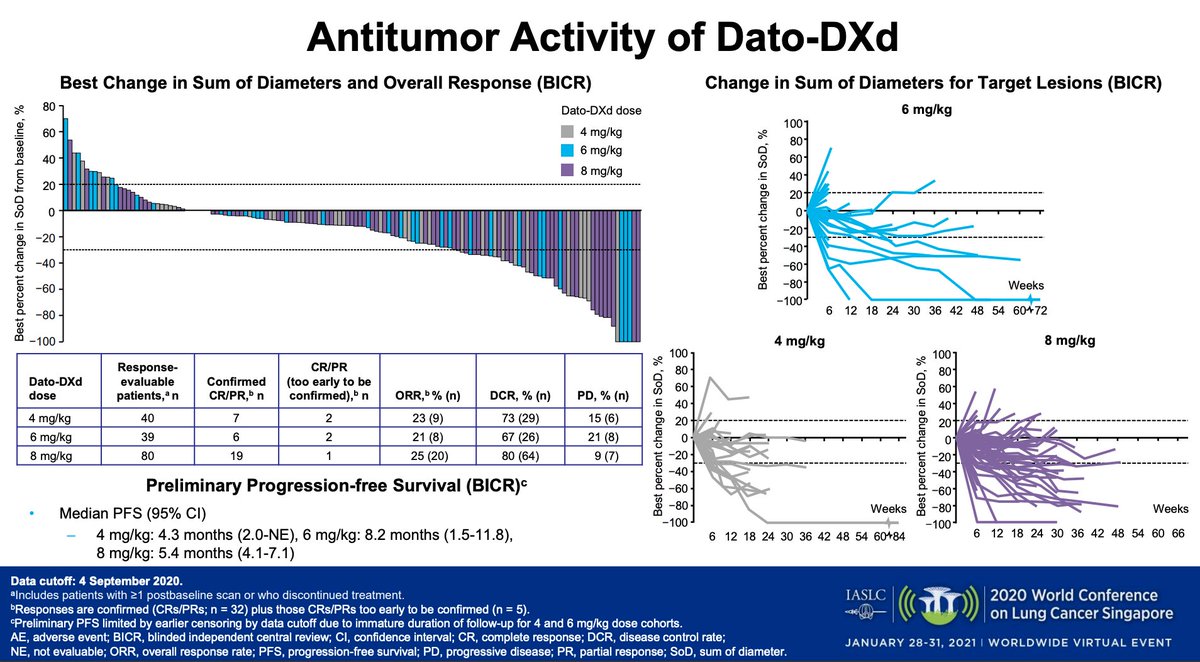
#WCLC20 Planned phase 3 TROPION-Lung01 (n=590) study will explore the 6mg/kg dose (agree that the 8mg, despite the high RR, seems too toxic). I think this is a promising compound, look forward to randomized data. #LCSM @IASLC @AlexSpiraMDPhD 



• • •
Missing some Tweet in this thread? You can try to
force a refresh