
#WCLC20 Results from the phase III non-inferiority J-AXEL study of salvage nab-paclitaxel vs docetaxel in NSCLC. Large study (n>500) with a 1:1 randomization. #LCSM @IASLC @OncoAlert 





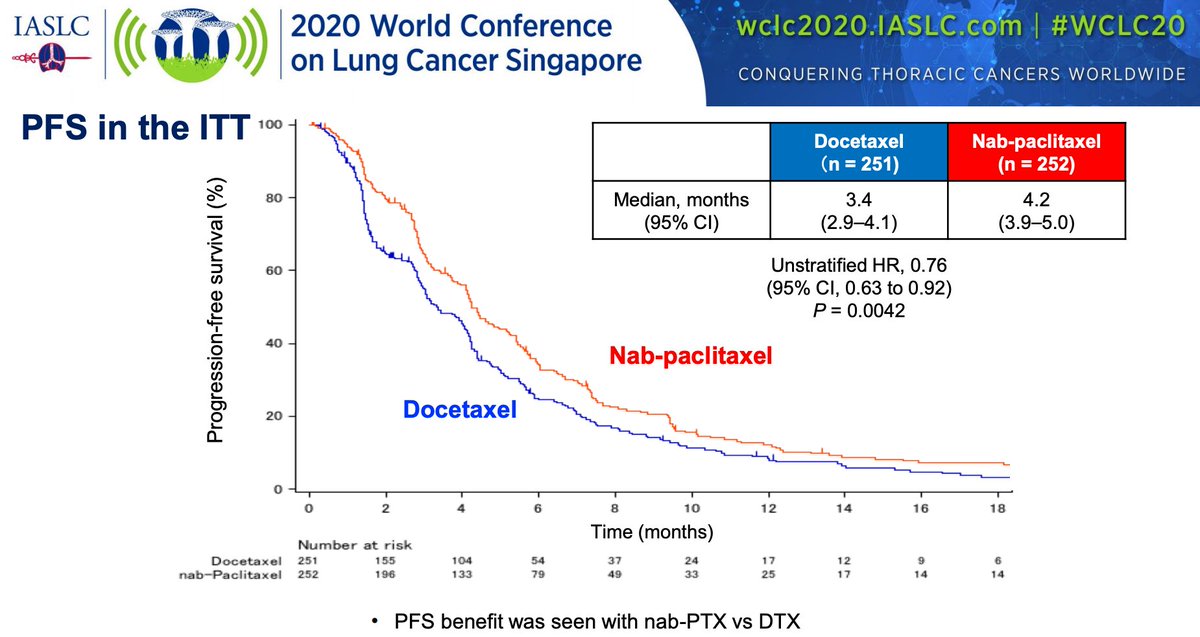
#WCLC20 Positive study! Shows non-inferiority in OS with nab-paclitaxel over docetaxel (median 16.2m vs 13.6m). PFS favors nab-pac (HR 0.76). #LCSM 


#WCLC20 RR favors nab-paclitaxel (30% vs 15%) and in this study, unlike the registrational trial, no real difference between squamous and non-squamous (both 30%, both better than docetaxel). #LCSM 

#WCLC20 Docetaxel had a higher rate of febrile NTP (22% vs 2%) and overall, nab-paclitaxel better tolerated (not sure about neuropathy below). I think the high RR and favorable toxicity are compelling but balanced by schedule (esp during COVID) and of course, cost. #LCSM 



• • •
Missing some Tweet in this thread? You can try to
force a refresh


































