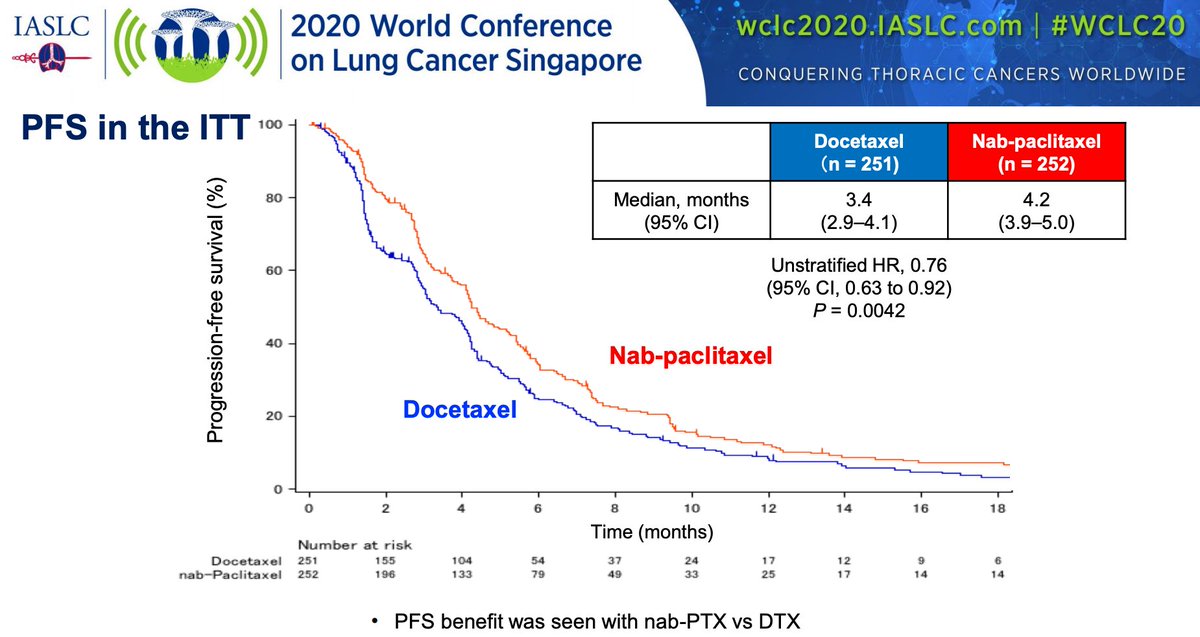
#WCLC20 Excited about this one: phase I results of the HER3 ADC patritumab deruxtecan (HER3-DXd; U3-1402) in #EGFR mutant #NSCLC presented by @HelenaYu923 #LCSM @IASLC @OncoAlert 

#WCLC20 Presentation here is on the combined dose escalation and expansion at the 5.6 mg/kg dose with #EGFR mutant NSCLC. Includes 56 evaluable patients. Median f/u 5 months. #LCSM @IASLC 





#WCLC20 Fairly heavily pretreated population with both TKI and chemotherapy and many with IO as well. Biopsy required for HER3 expression (violin plot on right) and nearly all were HER3 positive. #LCSM 

#WCLC20 Impressive responses given the number of different resistance mechanisms represented in this heavily pretreated population. 55% with treatment ongoing. Most with some reduction. #LCSM 

#WCLC20 Responses here tended to be quick, some very deep (CR noted). The reported RR was 25% with a DCR of 70%, time to response 2m, duration of response 6.9m. Encouraging data here. #LCSM 



#WCLC20 As with many ADCs, common toxicities include myelosuppression (28% G3 plt, 19% G3 ntp). Interstitial lung disease noted in 3 patients (5%). Phase 3 HERTHENA-Lung 01 study ongoing. Keep an eye on this agent to possibly fill a huge unmet need. #LCSM 



• • •
Missing some Tweet in this thread? You can try to
force a refresh