
Published just now in @ASCO_pubs JCO Global Oncology. The FIRST report of a population-based cancer registry from Nepal. It felt very meaningful to contribute to this #cancergroundshot project. ascopubs.org/doi/full/10.12… #globaloncology 

For the first time, we now have official data on the commonest cancers in Kathmandu by incidence and mortality in different subgroups of population. ascopubs.org/doi/full/10.12… 
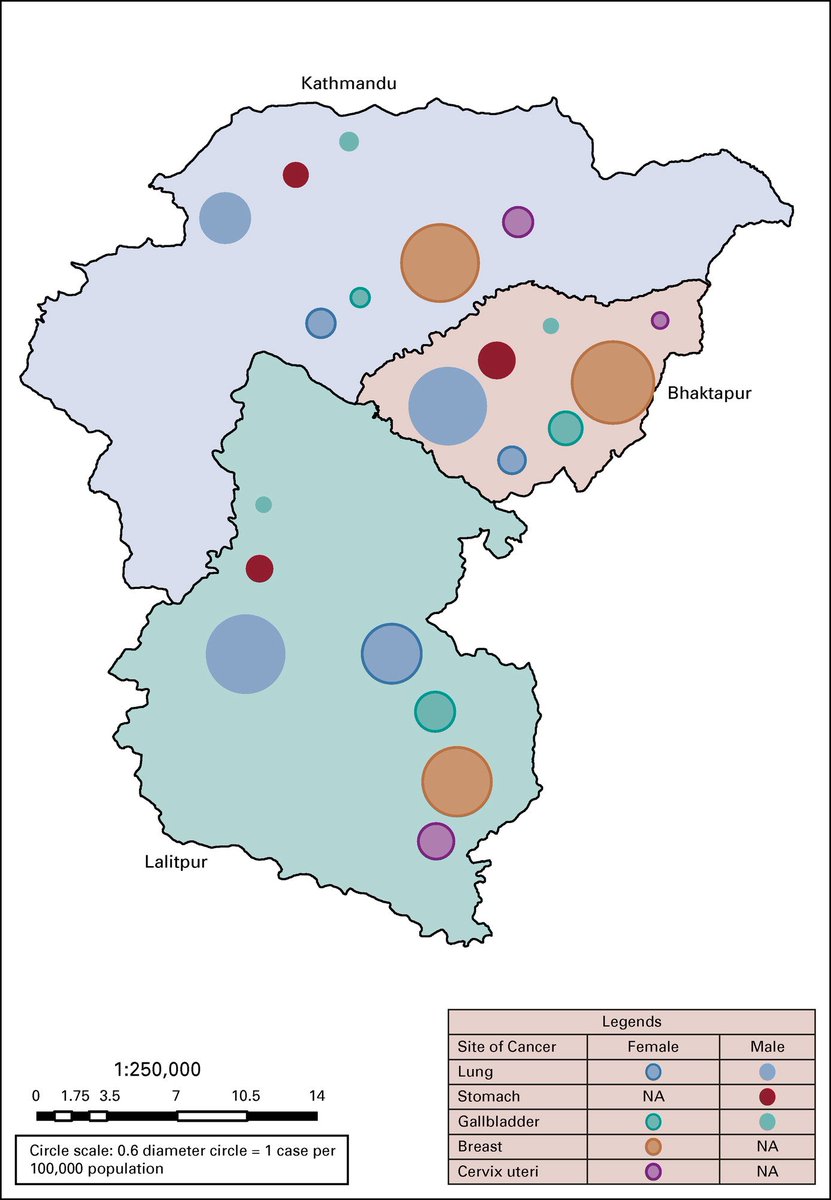
The population-based cancer registry has only just started in Nepal, and there are plenty of room for improvement. But I hope this provides the foundation for stronger work in the future. ascopubs.org/doi/full/10.12… 

Thank you Nepal Health Research Council for asking me to contribute to this project. It felt very meaningful to have been able to serve my country when she needed my expertise. ascopubs.org/doi/full/10.12…
• • •
Missing some Tweet in this thread? You can try to
force a refresh





