1/ $TMDX results are positive, but FDA's job is to question things every step of the way.
In spirit of being informed / keeping a balanced view, below are some FDA concerns:
- Trial design (single-arm study)
- $TMDX's subjective definition of extended-criteria hearts
...

In spirit of being informed / keeping a balanced view, below are some FDA concerns:
- Trial design (single-arm study)
- $TMDX's subjective definition of extended-criteria hearts
...


2/ - Extended-criteria hearts in EXPAND also include single-criterion >=4hr cross-clamp time, which actually make it somewhat overlap w/ PROCEED group
(i.e. imperfect definition extended-criteria heart)



(i.e. imperfect definition extended-criteria heart)


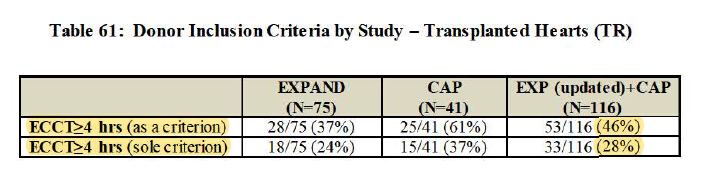
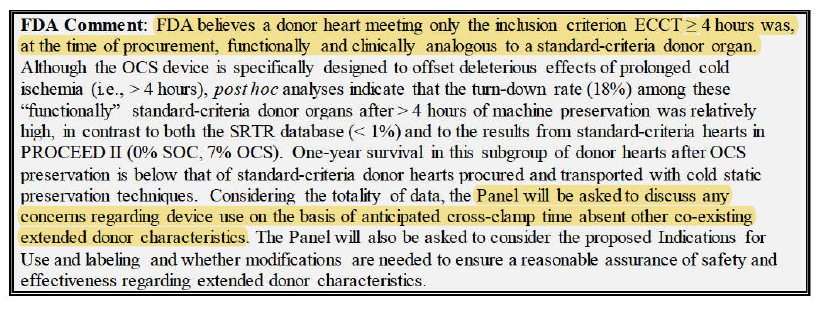
3/ - 65% as questionable performance goal (b/c $TMDX defined goal after benchmarking w/ old studies that achieved 22.6%-32.0%, but those studies didn't use standard definition of PGD and don't know proportion of extended-criteria pop in those studies)
...

...


4/ - PROCEED II's LT survival results (not homerun overall outcomes, but I guess $TMDX shows low cardiac death)
Side note:
PROCEED is supplemental. Panel on EXPAND.
Good thing EXPAND is doing better than OCS PROCEED. Also, there are arguably worse-condition hearts in EXPAND.

Side note:
PROCEED is supplemental. Panel on EXPAND.
Good thing EXPAND is doing better than OCS PROCEED. Also, there are arguably worse-condition hearts in EXPAND.
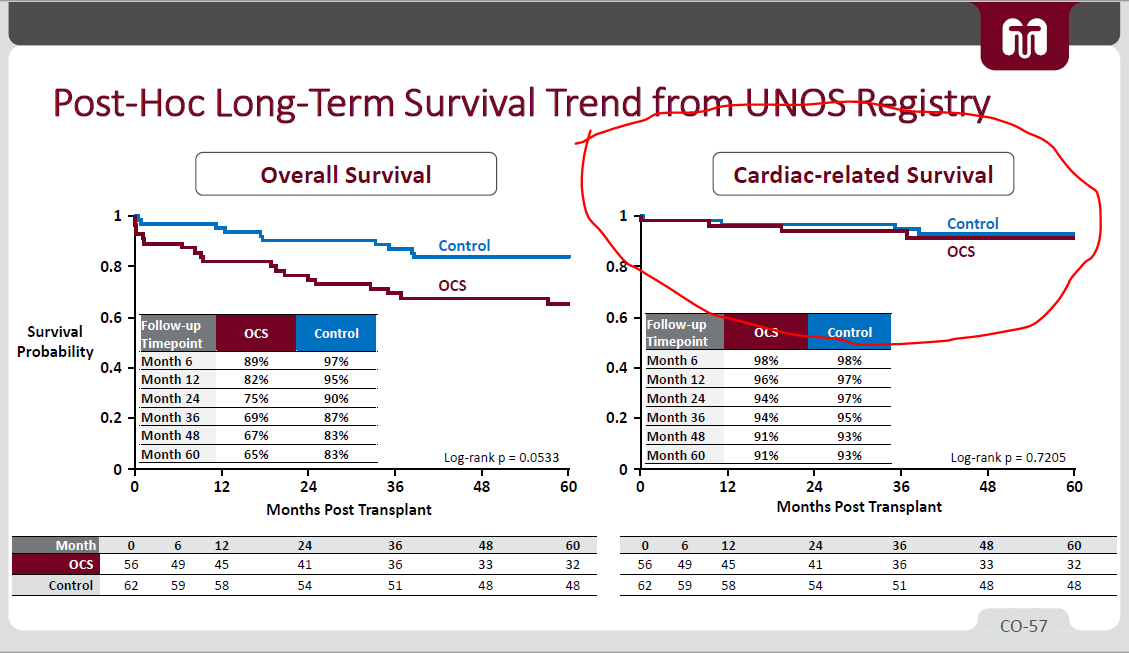

5/ - Using FDA's Piecewise exponential model, FDA thinks EXPAND survival rate might drop to rates similar to PROCEED
- (i) Use of MCS (Mechanical Circulatory Support) & (ii) ICU stay length both higher in EXPAND than PROCEED's OCS / Control
*but again, these are worse hearts


- (i) Use of MCS (Mechanical Circulatory Support) & (ii) ICU stay length both higher in EXPAND than PROCEED's OCS / Control
*but again, these are worse hearts



6/ Ultimately, $TMDX beat the 65% benchmark in primary endpoint, but MUST keep balanced view.
FDA can question how data came about / whether it is valid.
This will ultimately depend on Panel. Waleed, Schroder and others at meeting need to be on A-game and crush it.
FDA can question how data came about / whether it is valid.
This will ultimately depend on Panel. Waleed, Schroder and others at meeting need to be on A-game and crush it.
7/ I think it is crucial to emphasize that FDA Panel was never meant to be easy. This is normal.
As a rebuttal regarding single-arm trials, believe $TMDX will argue that it is unethical to randomize and use extended-criteria hearts w/ cold storage (can lead to death).
As a rebuttal regarding single-arm trials, believe $TMDX will argue that it is unethical to randomize and use extended-criteria hearts w/ cold storage (can lead to death).
8/ PROCEED II should in theory also be supplemental information, given there is no control group for EXPAND.
Main focus is on EXPAND results, not PROCEED II, which used a different OCS Solution (HTK Custodiol, which is extracellular i.e., low potassium)
Main focus is on EXPAND results, not PROCEED II, which used a different OCS Solution (HTK Custodiol, which is extracellular i.e., low potassium)
https://twitter.com/genghis_pon/status/1378937843121201155?s=20
9/ Personally not my job to guess FDA decision (even CEO Waleed keeps repeating that he's not in the position to do so).
However, $TMDX's OCS Heart should bridge well into DCD Hearts which is a revolutionary unlock in supply.
However, $TMDX's OCS Heart should bridge well into DCD Hearts which is a revolutionary unlock in supply.
10/ Leaving off w/ screenshot below. FDA mentions structure / setup of the trial, but let's not forget the worst survival rate is for unattended people on waitlists.
Believe $TMDX will give Tx surgeons more tools.
It will also give dying patients more options.
Believe $TMDX will give Tx surgeons more tools.
It will also give dying patients more options.

• • •
Missing some Tweet in this thread? You can try to
force a refresh