Schroder just started presenting at $TMDX Meeting
Schroder crushed it with his closing sentence for his segment saying OCS technology not only solves current quantity in the waitlist but can also allow an increase in waitlist size.
He literally said that there are plenty of good hearts out there - - just need to use OCS.
He literally said that there are plenty of good hearts out there - - just need to use OCS.
Chris Mullin (Independent Advisor) brought on by $TMDX reiterated the Piecewise FDA model is not valid nor reliable for long-term death extrapolation.
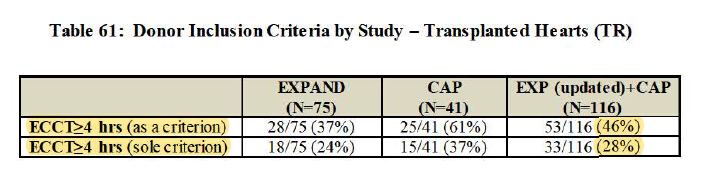
Regarding EXPAND + CAP overlap w/ PROCEED, Waleed emphasizing clearly EXPAND + CAP pool are much WORSE hearts than PROCEED and UNOS.
EXPAND + CAP hearts REFUSED for transplants 22x
PROCEED refused only TWICE
UNOS Cold Storage national only TWICE
EXPAND + CAP hearts REFUSED for transplants 22x
PROCEED refused only TWICE
UNOS Cold Storage national only TWICE

Ashish Shah, MD says $TMDX OCS can DOUBLE the heart transplant numbers.
He said no other technology can make this possible today.
He said no other technology can make this possible today.
Ashish Shah emphasizes UNOS today allows donor organs to travel <500mi w/ mean cold storage time of 3.2 hours.
For EXPAND, Distance in EXPAND is 912 miles and cross-clamp time of 7.2 hours.
He says he is very excited as a surgeon and that this is the future of Heart Transplant
For EXPAND, Distance in EXPAND is 912 miles and cross-clamp time of 7.2 hours.
He says he is very excited as a surgeon and that this is the future of Heart Transplant
Q&A time: When asked about lack of control group and which doctors thought single-arm made sense, Waleed started listing out a SLEW of names of Heart Failure doctors and Heart Surgeons.
It was a unanimous decision that can't do cold storage with EXPAND-quality hearts.
It was a unanimous decision that can't do cold storage with EXPAND-quality hearts.
Q&A (cont'd):
Waleed noted that in the single inclusion scenario of only >4 hours cross-clamp, Cardiac Death was ABSOLUTELY ZERO.
4 deaths were from completely other reasons, not from $TMDX causes.
Person who asked question looked satisfied with answer.
Waleed noted that in the single inclusion scenario of only >4 hours cross-clamp, Cardiac Death was ABSOLUTELY ZERO.
4 deaths were from completely other reasons, not from $TMDX causes.
Person who asked question looked satisfied with answer.
Dr. Selzman pushing a little hard.
Dr. Selzman asking Waleed about Lactate. Asked re: other heart function and other factors (not just lactate) and whether there are better ways assess a heart.
...
Dr. Selzman asking Waleed about Lactate. Asked re: other heart function and other factors (not just lactate) and whether there are better ways assess a heart.
...
Answer for above:
Waleed talked about return to sinus rhythm, contractability of the heart. Admits Lactate is key sign of ischemia, but he says surgeon needs to assess based on aortic pressure, coronary flow, and use discretion.
Waleed talked about return to sinus rhythm, contractability of the heart. Admits Lactate is key sign of ischemia, but he says surgeon needs to assess based on aortic pressure, coronary flow, and use discretion.
Waleed now just talked about $TMDX certification and program can educate surgeons well. He said can ask various KOL / leaders at transplant programs.
Dr. Kwon bringing question back to lactate again. Also concerned about blowup in cost in selection of donors and dry-run.
$TMDX said they are investing in program right beside user. Dry-runs are existing nature of Transplants and covered by commercial/CMS payers. etc.
$TMDX said they are investing in program right beside user. Dry-runs are existing nature of Transplants and covered by commercial/CMS payers. etc.
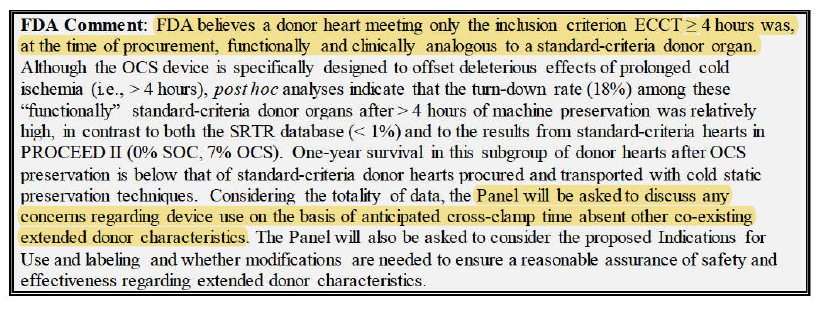
FDA section now coming up. Now the more bearish arguments coming up. Get ready.
FDA talking about how they wanted non-randomized concurrent control group.
FDA wanted severe and moderate PGD in primary endpoint, not just severe.
Cites conflict regarding cohort in primary analysis population b/c leaves out important ppl
FDA wanted severe and moderate PGD in primary endpoint, not just severe.
Cites conflict regarding cohort in primary analysis population b/c leaves out important ppl
FDA talking about no animal study. Waleed already addressed this earlier though in his segment.
FDA mentioned hard comparison in tissue injury as a result of the device because of no control.
FDA mentioned hard comparison in tissue injury as a result of the device because of no control.
Issues FDA will cover:
Study design
Study conduct
Definition of Extended-criteria hearts
Lactate as a metric
Survival
OCS Device Safety
Impact of OCS
(we kind of went over this with previous thread)
Study design
Study conduct
Definition of Extended-criteria hearts
Lactate as a metric
Survival
OCS Device Safety
Impact of OCS
(we kind of went over this with previous thread)
Still listening to this call but it’s late night in Bangkok. FDA is going through Presentation slide by slide and factually.
Can read the executive summary.
Stopping my real-time updates.
Can read the executive summary.
Stopping my real-time updates.

Gotta give it to the FDA though. These guys are very detail-oriented. Doing their job well.
Unfortunately for them though, without OCS, a whole new market of hearts will never be touched. And the thing is, we need more hearts.
Unfortunately for them though, without OCS, a whole new market of hearts will never be touched. And the thing is, we need more hearts.
Speaker 2: David Klassen (from UNOS) showing up!
Talks about Tx waitlists and says the numbers do not address the needs.
Klassen says major limitation is number of donors. He says DCD is changing that, and perfusion tech allows that.
Talks about Tx waitlists and says the numbers do not address the needs.
Klassen says major limitation is number of donors. He says DCD is changing that, and perfusion tech allows that.
He says there are new sources of donors as well as broader sharing.
He says "this sort of approach" (meaning warm perfusion i.e. $TMDX ) will be the way forward.
He says "this sort of approach" (meaning warm perfusion i.e. $TMDX ) will be the way forward.
Speaker 3: Sean Pinney, MD also showing up!!
He said OCS specifically will expand demand.
Allows utilization of higher risk organs.
Hearts that are usually discarded.
Hearts from older donors.
Hearts with LVH, diabetes, require resuscitation.
He says OCS gives confidence.
He said OCS specifically will expand demand.
Allows utilization of higher risk organs.
Hearts that are usually discarded.
Hearts from older donors.
Hearts with LVH, diabetes, require resuscitation.
He says OCS gives confidence.
Pinney says OCS is better than cold storage, expand donor pool, help patients.
Speaker 4: Gregory Couper, MD
- Vouches for integrity of data and safety of $TMDX trial
- Says there is a need for new tech
- Limited donor availability
- Extended-criteria donors will need to make up more % of volume to help everybody
- OCS lowers ischemic time
- Vouches for integrity of data and safety of $TMDX trial
- Says there is a need for new tech
- Limited donor availability
- Extended-criteria donors will need to make up more % of volume to help everybody
- OCS lowers ischemic time
Speaker 5: Jason Smith, MD... ALSO showing up!
- Participated in EXPAND trial
- Would like to speak in support of $TMDX
- Northwest area geographically challenged so elected to participate in EXPAND Trial
- Improve access to donors
- Participated in EXPAND trial
- Would like to speak in support of $TMDX
- Northwest area geographically challenged so elected to participate in EXPAND Trial
- Improve access to donors
- Experience = improved organ function, improved access, better performance of organs from GREAT distances (some >2K miles)
- Very positive response for entire program
- Strongly support the use of OCS more broadly
- Experience in EXPAND led them to participate in DCD Heart
- Very positive response for entire program
- Strongly support the use of OCS more broadly
- Experience in EXPAND led them to participate in DCD Heart
Speaker 6: Mani Daneshmand, MD (Duke, then Emory)
- Utilized OCS Heart AND Lung device
- Ability to provide and match organs that would otherwise be unutilized
- OCS overcomes a lot of issues
- Transplants could be done quicker and before recipients got very sick
- Utilized OCS Heart AND Lung device
- Ability to provide and match organs that would otherwise be unutilized
- OCS overcomes a lot of issues
- Transplants could be done quicker and before recipients got very sick
- Daneshmand used OCS at Duke and when he went to Emory, he couldn't use OCS
- Emory then got involved in DCD trial
- Daneshmand says he now has new hope and is very happy to be using OCS again
- Emory then got involved in DCD trial
- Daneshmand says he now has new hope and is very happy to be using OCS again
Speaker 7: Patrick Sullivan (CoFounder of Heart Bros Foundation)
- Organ transplant recipient
- Patients post transplant end up with PTSD
- LVAD experience (being plugged to the wall)
- Happy to speak on behalf of TMDX
- Organ transplant recipient
- Patients post transplant end up with PTSD
- LVAD experience (being plugged to the wall)
- Happy to speak on behalf of TMDX
Speaker 1: Nina Zeldes
- I missed her speech - I came back late from break!
- For sake of full disclosure, she was critical of program and apparently a negative review of TMDX
- The only negative one so far (she agreed with everything FDA said
- I missed her speech - I came back late from break!
- For sake of full disclosure, she was critical of program and apparently a negative review of TMDX
- The only negative one so far (she agreed with everything FDA said
Speaker 8/9/10: more transplant recipients!!
#8 thanked $TMDX and for opp to speak
#9 happy he can play baseball again ha (and also to get wait down)
#10 lived off of LVAD, then got DCD from MGH, zero complications a year out, back to golfing, hiking and playing w/ daughter
#8 thanked $TMDX and for opp to speak
#9 happy he can play baseball again ha (and also to get wait down)
#10 lived off of LVAD, then got DCD from MGH, zero complications a year out, back to golfing, hiking and playing w/ daughter
Waleed says OCS weeding out bad hearts that shouldn't be transplanted. Then you would see PGD in the time following the transplant. It's the opposite. Very low % especially compared to all other hearts.
Some controversy about animal studies. FDA got N=2 animals. Waleed said had more. FDA said they asked several times and she said Waleed said they didn't have to do comprehensive animal study. FDA says screenshot above was an abstract and done in 2006.
One panelist asked if haven't done animal study, then why go ahead with the PMA LOL
FDA said that there were clinical studies in Europe. $TMDX had evidence from Europe.
FDA said that there were clinical studies in Europe. $TMDX had evidence from Europe.
Dr. Demetris said there were edemas in areas but didn't see anything that was out of the ordinary.
Dr. Kwon summarizing that everybody's just trying to figure out if have myocardial damage.
Issue of lack of controls.
Dr. Kwon summarizing that everybody's just trying to figure out if have myocardial damage.
Issue of lack of controls.
In response to Al Stammers, Waleed said if apply EXPAND + CAP's 84% as extra utilization to currently unused DBD pop @ 8,400 - it will easily double transplants in U.S.
Al Stammers said might seem too high.
This became a TAM discussion for a second.
Al Stammers said might seem too high.
This became a TAM discussion for a second.
Schroder's back! Prior to OCS, he had PGD was 31%. Now it's 10%. He's not sure where 25% came from. He says that donor organ expansion is key.
Schroder: process of choosing appropriate donor is not A + B = C. Surgeons know donor risk factor. Also about taking risk for SPECIFIC recipients.
Some turndowns might be about lactate but it could also be about taking risk for SPECIFIC recipients. AGAIN, clinical judgement
Some turndowns might be about lactate but it could also be about taking risk for SPECIFIC recipients. AGAIN, clinical judgement
Dr. Kwon: PGD rigid 24hr too stringent? It lingers
Waleed: $TMDX through other endpoint captures up to 30 days.
Waleed: $TMDX through other endpoint captures up to 30 days.
Schroder: 44 y.o. donor, alochol abuse, downtime, usually it is turndown for 100% in country
In the past, Duke uses these donors. Ischemic time on the other side of Raleigh as long as $TMDX.
In the past, Duke uses these donors. Ischemic time on the other side of Raleigh as long as $TMDX.
FDA Q1: Discuss impact of design/study conduct issues on safety/effectiveness/benefit-risk
Pretty negative from many panelists re: design. Similar to going back to first step of trial b/c they feel this should be figured out. Panelists expressed frustration at sponsor & FDA.
Pretty negative from many panelists re: design. Similar to going back to first step of trial b/c they feel this should be figured out. Panelists expressed frustration at sponsor & FDA.
Lack of valid comparison. In general very TOUGH question. Also how to treat indications?
Dr. Bonde actually supported $TMDX and said there are no other options.
Dr. Selzman said if add in moderate PGD, will still be >65% guideline so should also address other questions.
Dr. Bonde actually supported $TMDX and said there are no other options.
Dr. Selzman said if add in moderate PGD, will still be >65% guideline so should also address other questions.
FDA Q2: EXPAND inclusion criteria: 40 were single-inclusion. 18 >=4hr. Discuss overlap.
Bonde is thinking about 'flying blind with the cold storage'.
Dr. Moon says he thinks results are worse on TMDX, BUT he doesn't know if it's because it's worse patients or b/c of OCS.
Bonde is thinking about 'flying blind with the cold storage'.
Dr. Moon says he thinks results are worse on TMDX, BUT he doesn't know if it's because it's worse patients or b/c of OCS.
Dr. Allen terribly conflicted. A lot of hearts wasted. But if approved, OCS will be used for all other Tx, which is why he goes back to PROCEED. Afraid of scoop and run philosophy. How to make sure it is used in indications? Indication Creep
If can control, Dr. Allen is in.
If can control, Dr. Allen is in.
Dr. Kwon thinks study criteria too liberal.
Fernando Aguel FDA said question is about current indication.
Catherine Wentz FDA repeated that question is about overlap.
Fernando Aguel FDA said question is about current indication.
Catherine Wentz FDA repeated that question is about overlap.
FDA Q3: Reliability of lactate as determinant?
Dr. Moon has questions about lactate (he's been talking about animal studies OVER and OVER)
Dr. Allen not hung up on lactate levels. Have to hang hat on something
Dr. Moon has questions about lactate (he's been talking about animal studies OVER and OVER)
Dr. Allen not hung up on lactate levels. Have to hang hat on something
Dr. Bonde thinks lactate is just an indicator but other factors will decide. Centers will utilize a donor heart even at lactate level 6 or 7.
Final general feeling that lactate shouldn't be sole determinant.
Final general feeling that lactate shouldn't be sole determinant.
FDA Q4: PROCEED II & EXPAND Survival
Dr. Connor thinks FDA survival model is VERY sensitive especially using low sample size. Less concerned about LT than 0-6mos. Just not enough data to make LT projections.
Dr. Yeh doesn't know what to compare EXPAND to.
Dr. Connor thinks FDA survival model is VERY sensitive especially using low sample size. Less concerned about LT than 0-6mos. Just not enough data to make LT projections.
Dr. Yeh doesn't know what to compare EXPAND to.
Dr. Moon reiterates this should be used just for marginal donor.
Dr. Selzman says assumptions are being made. With number of patients, can't extrapolate LT data.
Dr. Bonde talks about MCS causing non-cardiac death
Dr. Selzman says assumptions are being made. With number of patients, can't extrapolate LT data.
Dr. Bonde talks about MCS causing non-cardiac death
FDA Q4B: Strengths & Limitations of Comparison and whether EXPAND indicate benefit of shorter wait time
Dr. Allen says you won't offer high-risk heart to a not-so-unhealthy waitlist recipient. He's afraid of taking a bad heart and extending it to a somewhat ok recipient.
Dr. Allen says you won't offer high-risk heart to a not-so-unhealthy waitlist recipient. He's afraid of taking a bad heart and extending it to a somewhat ok recipient.
Dr. Selzman says if you have more organs, it will lower durable LVADs. LVADs get people very sick and will be taken off the list. Selzman wants ppl to keep this perspective. Can shorten wait time for Group O BloodType.
FDA Q4C: Safety / effectiveness for donor hearts that considered non-standard?
Dr. Selzman supports $TMDX. Out of body time great. Results speak for themselves. Selzman says he hasn't seen anything that shows EXPAND doesn't work.
Dr. Selzman supports $TMDX. Out of body time great. Results speak for themselves. Selzman says he hasn't seen anything that shows EXPAND doesn't work.
Dr. Katz says if PGD go down to 8% then it benefits, but MCS can be a problem.
Dr. Kwon says he doesn't think device moved needle enough to be able to say safe and effective.
Dr. Bonde thinks $TMDX proved that OCS is safe and organs work.
DIVERSE opinions here.
Dr. Kwon says he doesn't think device moved needle enough to be able to say safe and effective.
Dr. Bonde thinks $TMDX proved that OCS is safe and organs work.
DIVERSE opinions here.
FDA Q5: Pathophysiologic / pathologic observations + myocardial damage w/ donor hearts
Dr. Borer say numbers are too small to really know positive or negative predictive value.
Dr. Selzman says it provides element of safety for population being studied.
Diverse opinions again
Dr. Borer say numbers are too small to really know positive or negative predictive value.
Dr. Selzman says it provides element of safety for population being studied.
Diverse opinions again
FDA Q6a: Discuss inclusion criteria and whether it will increase heart utilization and acceptable survival?
Dr. Moon says >=4hr makes sense.
Dr. O'Conner says afraid of indication creeping.
Dr. Moon says >=4hr makes sense.
Dr. O'Conner says afraid of indication creeping.
Dr. Bonde thinks distance on its own should be criteria. Distance + risk factor too. Risk can be judged by higher distance.
More people got organs b/c of distance covered by OCS.
More people got organs b/c of distance covered by OCS.
Dr. Katz won't increase risk of recipient waiting at home by giving Extended Criteria Heart. CLINICAL JUDGEMENT
Dr. Selzman says depends on many factors but will be too complicated formula to create.
Dr. Selzman says depends on many factors but will be too complicated formula to create.
FDA Q6b: Going through by individual inclusions to judge appropriateness
Dr. Allen says it's not simple. There are comorbidities. Can't just say what checks and doesn't check the box.
Dr. Kwon thinks 2hr. ischemic time plus risk factors are arbitrary.
Dr. Allen says it's not simple. There are comorbidities. Can't just say what checks and doesn't check the box.
Dr. Kwon thinks 2hr. ischemic time plus risk factors are arbitrary.
FDA Q7: Benefit/Risk
Dr. Moon: >4hr. off-limit for the most part. OCS better than leaving organ in the bucket.
Dr. O'Conner: Should use PROCEED II study to be informed on Safety but agree w/ Dr. Moon. One can find a sweet spot for OCS use where >4hr. + risk factors
Dr. Moon: >4hr. off-limit for the most part. OCS better than leaving organ in the bucket.
Dr. O'Conner: Should use PROCEED II study to be informed on Safety but agree w/ Dr. Moon. One can find a sweet spot for OCS use where >4hr. + risk factors
Dr. Cigarroa: Find it challenging to not include PROCEED II study. Difficulty in interpreting EXPAND + EXPAND CAP because concentration in 1-2 sites
Dr. Allen: Can't ignore PROCEED II study because they've done the trial. Actually wish they hadn't done it. Would be easier.
Dr. Allen: Can't ignore PROCEED II study because they've done the trial. Actually wish they hadn't done it. Would be easier.
Dr. Allen (Cont'd): OCS can have lower outcome than standard of care per PROCEED. Also, how well can FDA put controls? If can, then it's easier to think through.
FDA Q8: Post Approval Study
Can still run an observational trial (find ppl on Waitlist who would want OCS) compare vs. ppl who refuse and use as control.
Dr. O'Conner thinks bar is way too low. Need high #.
Expect a very rigorous post approval study (if we get to that)
Can still run an observational trial (find ppl on Waitlist who would want OCS) compare vs. ppl who refuse and use as control.
Dr. O'Conner thinks bar is way too low. Need high #.
Expect a very rigorous post approval study (if we get to that)
MEETING FINAL WORDS:
Waleed deferring to Dr. Milano from Duke.
Milano refers to Cold Storage as primitive and far from gold standard.
Waleed deferring to Dr. Milano from Duke.
Milano refers to Cold Storage as primitive and far from gold standard.
VOTE:
Q1: Safety for patients in proposed indication
Yes 9 / No 7 / Abstain 2
Q2: Effectiveness for patients in proposed indication
Yes 10 / No 6 / Abstain 2
Q3: Benefits outweigh the risk for patients in proposed indication
Yes 12 / No 5 / Abstain 1
Q1: Safety for patients in proposed indication
Yes 9 / No 7 / Abstain 2
Q2: Effectiveness for patients in proposed indication
Yes 10 / No 6 / Abstain 2
Q3: Benefits outweigh the risk for patients in proposed indication
Yes 12 / No 5 / Abstain 1

Thanks everybody who followed the thread. I might have transcribed some things wrong because I'm doing it live. Please read actual transcript when it comes out instead.
As I previously said, there is a massive need for organs. They will be stringent. But seems they approved
As I previously said, there is a massive need for organs. They will be stringent. But seems they approved
APPROVE ABOVE MEANING PANEL VOTE YES.
NOT FDA. FDA COMES OUT LATER.
NOT FDA. FDA COMES OUT LATER.
Dr. Moon (Mr. We need an Animal Study) voted YES on all 3 questions...
This is SO HARD to guess results for. Simply can't even guess or you're just making things up.
This is SO HARD to guess results for. Simply can't even guess or you're just making things up.
• • •
Missing some Tweet in this thread? You can try to
force a refresh