
In Nov we learned that the 1st mRNA vaccine was 95% effective for COVID. Since then the # of people who’ve died has more than doubled. Yesterday's #TRIPS news is a start. Here are next steps @joebiden must take to end the pandemic: 🧵
We have developed a comprehensive plan on how we could rapidly scale mRNA vaccine production and have shared it with the administration since Feb. More than a 100 days into his admin, this president still has not developed a plan for global vaccination. prep4all.org/news/hit-hard-…
And while committing to negotiate a TRIPS waiver is something, @joebiden needs to take more immediate action *today*. There are three actions Biden, with just his phone and pen, could do today to put the world on the path to global vaccination. 3/n
NO 1. Commit now to strengthening the raw material supply chain. One of the myths we see being perpetuated by the pharmaceutical industry is that a TRIPS waiver would weaken the raw materials supply chain. The exact opposite is true. 4/n
Take the custom cationic (positively charged) lipids used in mRNA vaccines. These fat like molecules are attracted to the negatively charged mRNA molecules, allowing a lipid nanoparticle to form around the RNA core during the nanoprecipitation process. 5/n
The problem is that, right now, very few companies are making these custom molecules & there are shortages. And a big problem there is intellectual property. Let’s look at SM-102, the cationic lipid used in Moderna’s COVID-19 vaccine. 6/n
SM-102 is a small molecule (molecular weight: ~700 daltons). Right now, only a single company (@CordenPharma) is making SM-102 for @moderna_tx. And there have been severe shortages of this molecule as a result. 7/n 
Surely companies in the global south can make this molecule since they produce most of the global supply of API and excipients already. But any company who started manufacturing such a molecule would be infringing on a patent that protects it. 8/n
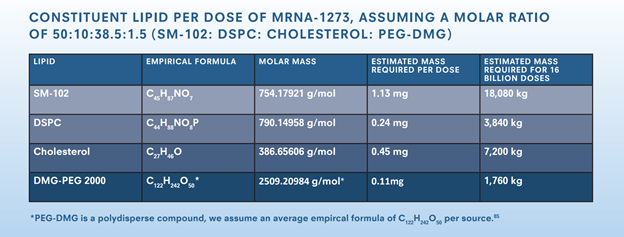
As our analysis indicates (pg. 34 of the report), even assuming 100% encapsulation efficiency, the world will need *alot* of SM-102. We estimate a *minimum* of 18 metric tons will be needed. 9/n 
But we have seen when other companies are allowed to make cationic lipids, they can rapidly scale. @Evonik, a German specialty company, was able to scale lipid production in just 8 weeks. 10/n biopharminternational.com/view/evonik-de…
Biden can issue, today, an open tender for the US government to purchaseSM-102 and utilize a law, 28 U.S.C. §1498, that allows any company to use patents without worrying about infringement liability. This is known as government patent use. 11/n
(If you want to learn more about 28 U.S.C. §1498 and government patent use--sometimes known as “compulsory licensing”--read our user manual on the law, edited by the great @cmorten2 at @nyulaw) 12/n prep4all.org/news/1498guide
This would stimulate manufacturing capacity for SM-102 and signal to other countries that the US approves of government patent use during this emergency. So if govts can help companies get around patents, why do we need a TRIPS waiver? 13/n
While the TRIPS agreement explicitly allows government patent use, it explicitly *prohibits* the export of goods made using government patent use to other countries (see article 31(f) of the agreement). 14/n
So even without a TRIPS waiver, the US government can use §1498 to allow any company in the US capable of making SM-102 (or any other patented product) to make, but it couldn’t *export it* to e.g. India. That’s what a TRIPS waiver unlocks. 14/n
NO 2: @joebiden must help scale public production of mRNA vaccines. A big issue in increasing vaccine access is the myth that rapid production scale up is impossible, and that we can’t make/distribute those vaccines in LMICs. 15/n
Before we go into the weeds, let's address the most common question we get: if we could rapidly and dramatically increase mRNA vaccine production, why isn’t pharma scaling up production? 16/n
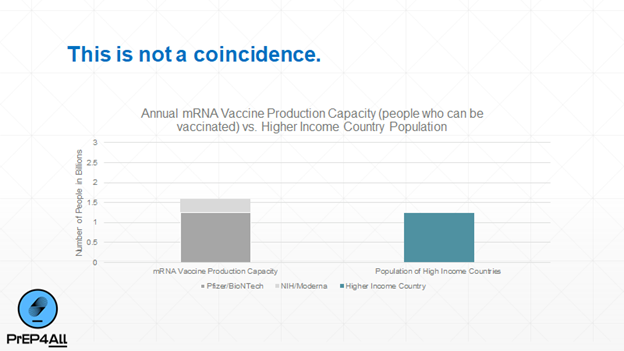
The answer: they are not incentivized to. This is a classic example of “market failure”. One thing that everyone agrees on is that ramping up vaccine production to the scale we need would cost billions of dollars. 17/n
And almost all of that additional capacity would serve LMICs, who would reasonably expect that the vaccine would be sold to them at cost (about $2 a dose) — rather than the ~10x markup that Moderna and Pfizer charge. 18/n
Once the pandemic is under control, the need for such massive vaccine capacity likely will drop. Remember, in non-pandemic times, total global demand for ALL vaccines combined is btw 3.5 - 5 billion doses py, according to @WHO. 19/n
The companies have already built enough capacity to vaccinate rich countries. They aren’t going to invest billions to serve demand that is not going to be very profitable and will disappear once the pandemic is brought under control. 20/n 
And @JoeBiden could do this *today*. In the rescue package passed in March, Congress allocated US$16 billion for vaccine manufacturing scale up to the Biden admin. Almost *none* of that has been spent. 21/n
The core of our plan relies on creating mRNA vaccine production plants that are owned by local governments (both in the US & around the world) but operated by experienced entities that know how to make vaccines. 22/n
How much would this cost? Estimates put the cost for mRNA vaccine plants that could produce 16 billion doses per year — enough to vaccinate the entire global population in a single year — at less than US$4 billion.
mdpi.com/2076-393X/9/1/…
mdpi.com/2076-393X/9/1/…
Even using the costs that @LonzaGroup used to build the first of a kind initial plants for the @moderna_tx, and assume zero economics of scale, we are still at less than US$13 billion for plants that produce 16 billion doses per year. 24/n
This is less than Congress already gave @joebiden in the rescue package. @joebiden could build plants around the world *with the money already appropriated by Congress* to vaccinate the entire world in a year. 25/n
And this could be done fast. At the start of the pandemic, there was ZERO industrial scale capacity for mRNA vaccines. Within 10 months, we had a multi-billion dose per year capacity built from scratch. 26/n
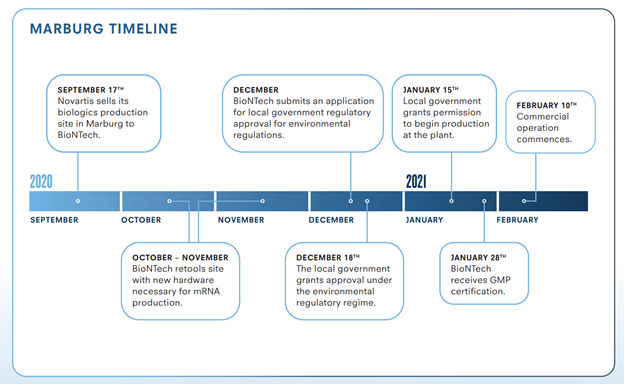
Companies retooled old factories into mRNA vaccine factories in under 6 months, e.g. BioNTech group’s conversion of Novartis’s old Marburg plant in Germany: Within six months, BioNTech was producing mRNa vaccines for Germany & Turkey. 27/n 
That brings us to NO 3: by creating publicly owned manufacturing facilities here and around the world, we create a workforce that could assist in teaching others how to make the vaccine. 28/n
This is similar to a model already used by the US government for technology transfer for pandemic influenza vaccines. 29/n medicalcountermeasures.gov/barda/influenz…
Since @joebiden has taken office, more than 1 million people around the world have died from COVID-19. This is no time to dither — every day we wait to act is another 10,000 lives lost. 30/n
• • •
Missing some Tweet in this thread? You can try to
force a refresh





