Here’s a plan to get India 10-20% extra COVISHIELD vaccines doses overnight!
How could this be possible? Read on to find out.
🧵 1/
Antibody Responses After a Single Dose of ChAdOx1 nCoV-19 Vaccine in HCWs Previously Infected with SARS-CoV-2 medrxiv.org/content/10.110…
How could this be possible? Read on to find out.
🧵 1/
Antibody Responses After a Single Dose of ChAdOx1 nCoV-19 Vaccine in HCWs Previously Infected with SARS-CoV-2 medrxiv.org/content/10.110…
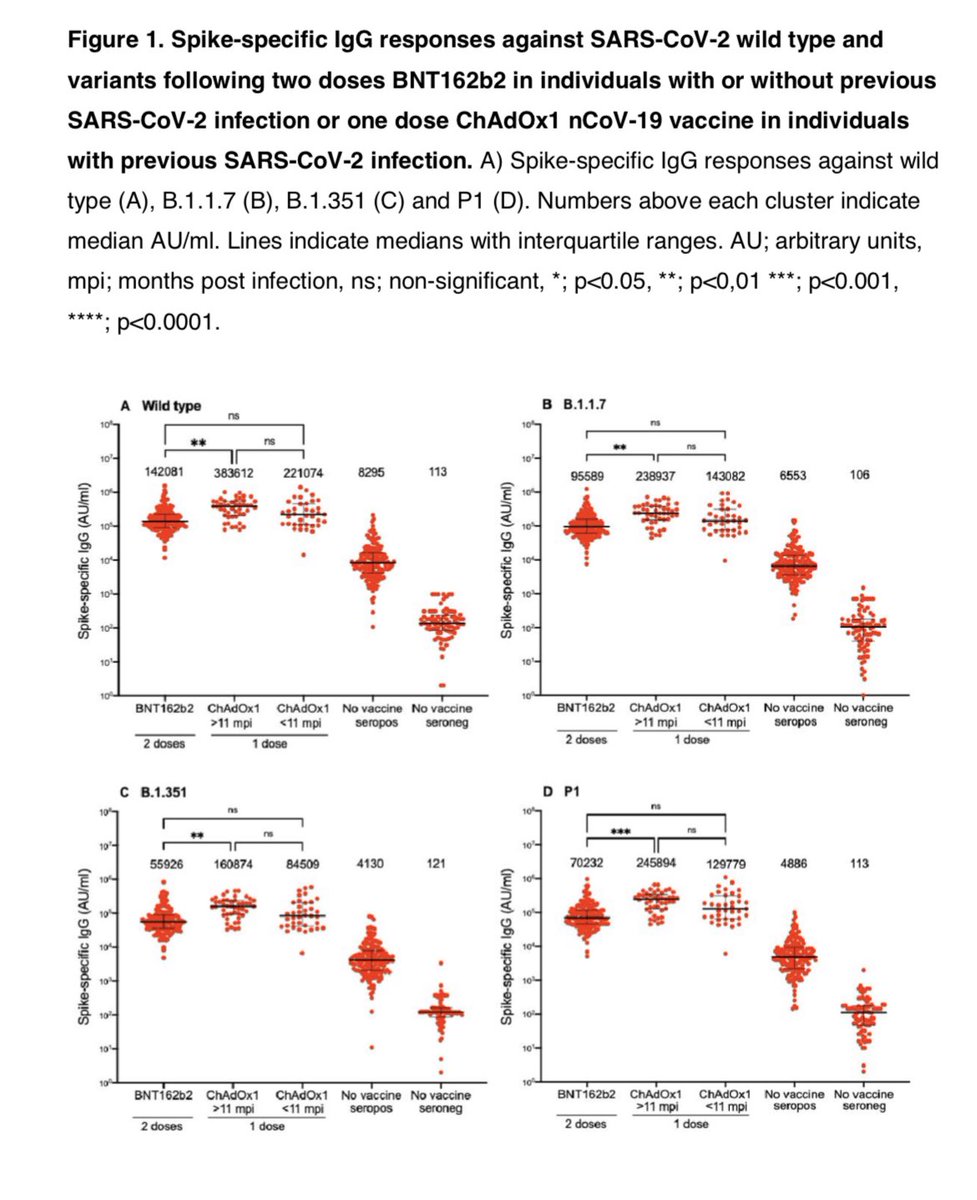
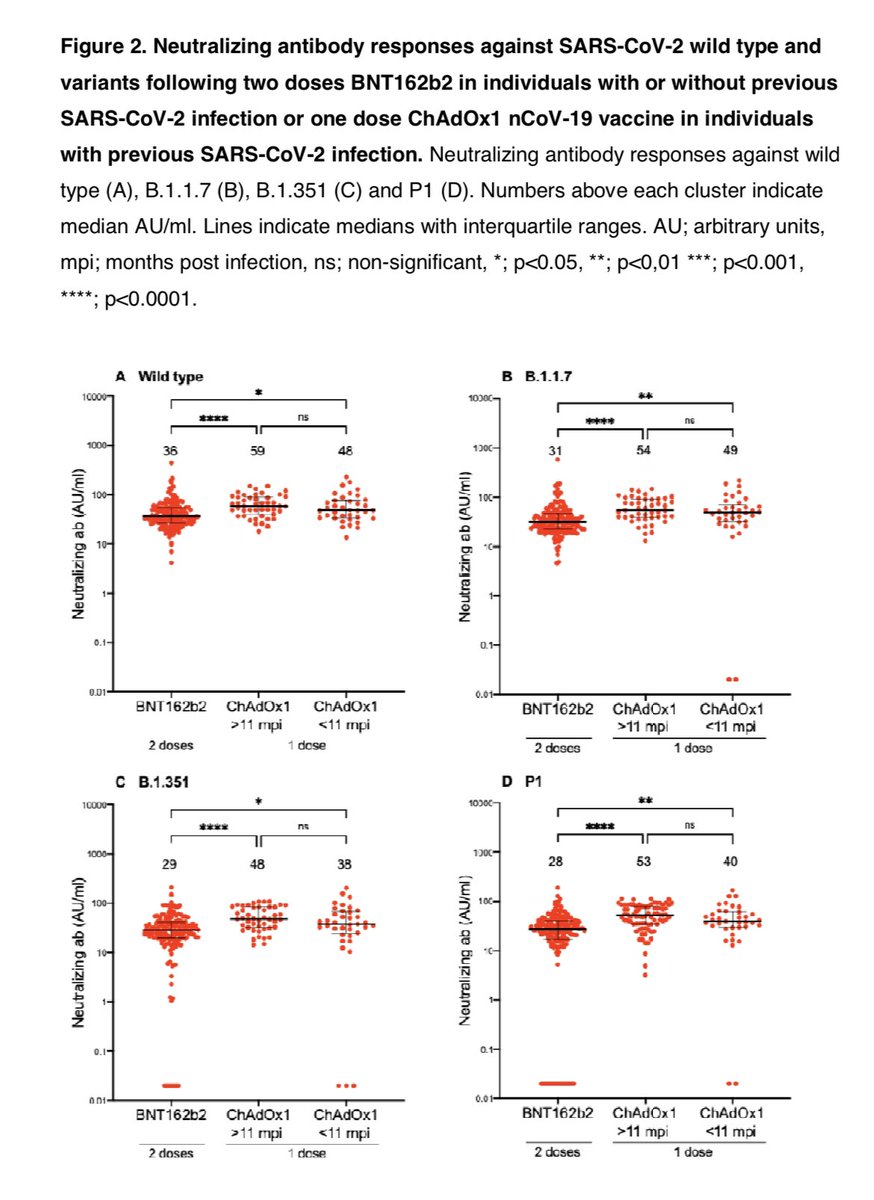
A study found that HCWs who had recovered from COVID-19 up-to 11 months prior responded incredibly to a single dose of AstraZeneca vaccine (COVISHIELD).
They had neutralizing antibodies against wild type virus but also neutralized P.1 & B.1.351 (immune escape variants)!
2/
They had neutralizing antibodies against wild type virus but also neutralized P.1 & B.1.351 (immune escape variants)!
2/
In fact those who had COVID-19 in the past and took a single dose of AZ (COVISHIELD) had higher neutralizing antibodies than those who took 2 doses of Pfizer mRNA vaccine! That’s right - past infection plus 1 dose AZ higher nAbs than 2 doses of Pfizer!
3/
medrxiv.org/content/10.110…
3/
medrxiv.org/content/10.110…
A single dose of COVISHIELD in those who had COVID-19 upto 11 months prior produces an amazing response. They can neutralize even the most concerning immune escape variant B.1.351 (variant identified by South African researchers)! Response is better than 2 doses of Pfizer.
4/
4/

A single dose of AZ (COVISHIELD) after natural infection upto 11 months prior has higher nAbs than 2 doses of Pfizer. We know 2 doses of Pfizer produce higher nAbs than 2 doses of COVISHIELD.
Change guidelines! Give those with past COVID-19 just 1 dose of COVISHIELD.
5/

Change guidelines! Give those with past COVID-19 just 1 dose of COVISHIELD.
5/
We always have to weight the benefit of any vaccine against the risk. Those who have had COVID-19 are fully protected after 1 dose of COVISHIELD. Why would we give them another dose? What value dose it provide them? There’s the rare risk of dangerous clots (VITT).
6/
6/
In these individuals the second dose doesn’t add much benefit (they already have a better response than 2 doses of Pfizer). So why should they take any risk? Even a negligible risk is unacceptable when there isn’t tangible benefit! So from their pint of view 1 dose makes sense
7/
7/
We have a catastrophic vaccine shortage in our country.
30-40% of people have likely had past infection. If we give them just a single dose of COVISHIELD we save 15-20% vaccine doses! Every dose saved is 1 extra dose we desperately need!
8/
30-40% of people have likely had past infection. If we give them just a single dose of COVISHIELD we save 15-20% vaccine doses! Every dose saved is 1 extra dose we desperately need!
8/
So from the individual point of view 1 dose makes more sense. From the public health point of view 1 dose makes sense.
From the govt. point of view 1 dose makes sense as cost to state is reduced.
We need these extra doses so badly. 15-20% more doses overnight!
9/
From the govt. point of view 1 dose makes sense as cost to state is reduced.
We need these extra doses so badly. 15-20% more doses overnight!
9/
How to know who was infected in past? Antibody tests!
Won’t that be too cumbersome? Use rapid tests just before vaccination!
What about false negative?
Irrelevant - someone gets an extra 2nd dose.
What about false positive? Orthogonal confirmation with a 2nd rapid test!
10/
Won’t that be too cumbersome? Use rapid tests just before vaccination!
What about false negative?
Irrelevant - someone gets an extra 2nd dose.
What about false positive? Orthogonal confirmation with a 2nd rapid test!
10/
We can use rapid tests for anti-RBD antibodies prior to first dose. That way no one gets sent back without 1 dose.
Here’s a thread to explain the plan in detail:
11/
Here’s a thread to explain the plan in detail:
11/
https://twitter.com/swapneilparikh/status/1391311579912695808
I want to clarify a COI
I consulted on R&D for a company that developed a rapid anti-RBD test. I DO NOT earn any royalties and WOULD NOT finically benefit from sales of this test. I explained this plan to the company months back, they understood the importance of such a test
12/
I consulted on R&D for a company that developed a rapid anti-RBD test. I DO NOT earn any royalties and WOULD NOT finically benefit from sales of this test. I explained this plan to the company months back, they understood the importance of such a test
12/
Adding @sailorrooscout 🧵
Also want to point out this is a pre-print. It has not been peer reviewed. The sample size is small. BUT we are in a moment of national crisis. We need to be bold in our response. We can implement pre dose 1 testing and measure response post dose 1
13/
Also want to point out this is a pre-print. It has not been peer reviewed. The sample size is small. BUT we are in a moment of national crisis. We need to be bold in our response. We can implement pre dose 1 testing and measure response post dose 1
13/
https://twitter.com/sailorrooscout/status/1392449876957536256
If plan doesn’t work at worst we added some pre-dose 1 rapid antibody tests. We would know based on the post dose 1 serology if the plan was working or not. If it doesn’t work we can always give the 2nd dose. If it’s works we get an extra 15-20% COVISHIELD doses. No downside!
14/
14/
• • •
Missing some Tweet in this thread? You can try to
force a refresh