
Provided COVAXIN single dose efficacy is >70%, models support delaying COVAXIN 2nd dose except for 65 years +
We should have had quality Ph3 data and real world data on COVAXIN by now. Unbelievable that we’re still doing guess work.
🧵
1/
We should have had quality Ph3 data and real world data on COVAXIN by now. Unbelievable that we’re still doing guess work.
🧵
1/
Modeling study helps us understand lives saved with delayed second dose of Covaxin.
Assuming vaccination rate of 0.3% of population / day. The total cumulative mortality on day 180 is seen to be lower as below depending on efficacy. Makes sense if efficacy >70%
2/
Assuming vaccination rate of 0.3% of population / day. The total cumulative mortality on day 180 is seen to be lower as below depending on efficacy. Makes sense if efficacy >70%
2/

Delayed second dose except for 65+ allows us to save lives but reduces the risk due to uncertain efficacy. Bottom line if COVAXIN single dose efficacy is >70% this approach makes sense given India’s daily vaccination rate. We’re unlikely to ever hit 1% population / day
3/
3/
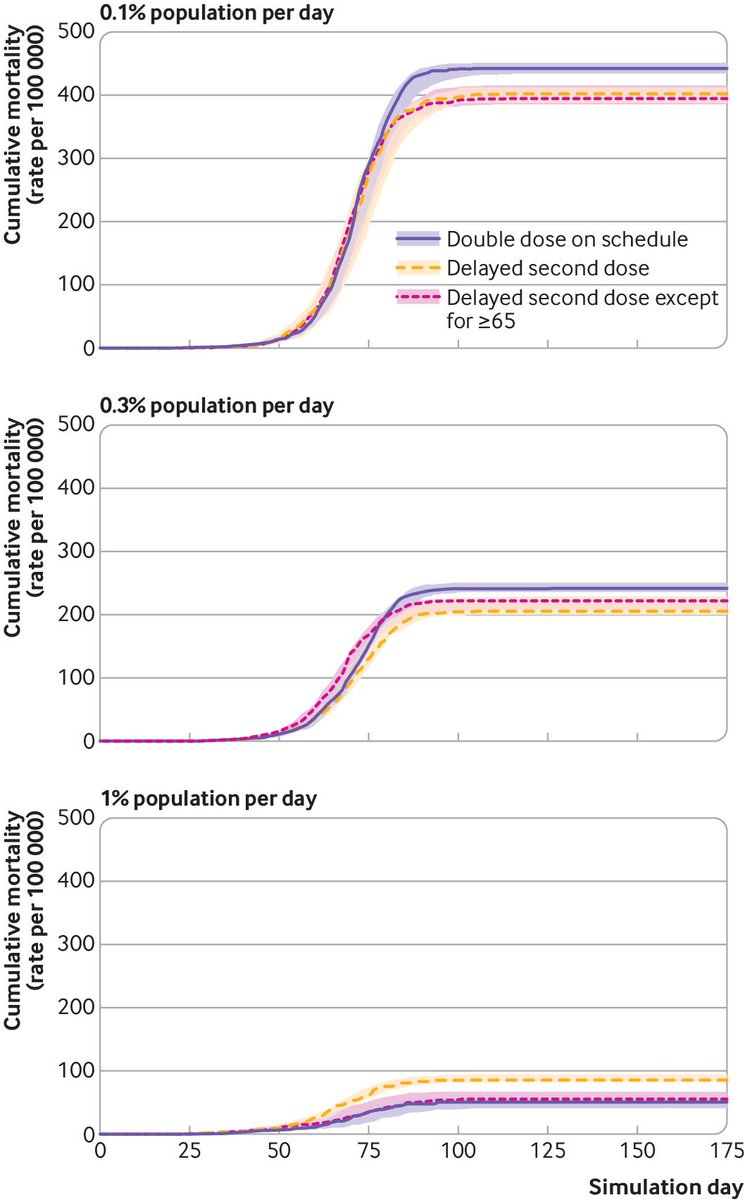
Under specific conditions a decrease in cumulative mortality, infections, & hospitalization can be achieved when 2nd dose is delayed. Most significant when the 2nd dose delayed in <65 years, with 2nd dose prioritized in time for >65.
bmj.com/content/373/bm…
4/
bmj.com/content/373/bm…
4/
“The conditions in which these benefits were observed included the first dose vaccine efficacy being above 70% and vaccination rates remaining below 1% of the population per day.”
Bharat Biotech & ICMR have (hopefully) Ph3 and real world data for single dose efficacy
5/
Bharat Biotech & ICMR have (hopefully) Ph3 and real world data for single dose efficacy
5/
Delayed second dose except for 65+ with COVAXIN will allow for benefit of saving more lives while reducing risk by ensuring timely dosing for the high risk group (65+).
In parallel we can study the benefit in the 45-65 age group of delayed dosing on lives saved.
6/
In parallel we can study the benefit in the 45-65 age group of delayed dosing on lives saved.
6/
This is not an ideal option. This is a Hail Mary because we’re in a catastrophic and largely avoidable vaccine shortage scenario. This is crisis time plan to save lives. It is not with out risk. Prioritizing 65+ for 2nd COVAXIN dose on time takes away a lot of the risk.
7/
7/
Earlier many, including myself, incorrectly believed that such delays might result in increased risk of immune escape variants. This was likely incorrect, and it’s probable that single dosing may actually reduce the risk of immune escape variants.
8/
8/
We need to administer as many first doses as possible ASAP. We should delay the second dose of COVAXIN to 12 weeks for all below 65 years. We should study lives saved in parallel. It should never have come to this. It’s an abysmal failure that we’re here but we’re here.
9/
9/
• • •
Missing some Tweet in this thread? You can try to
force a refresh





