🧬 Life as we don't know it 🧫
Exotic solvents & life's building blocks are among the more speculative
#astrobiology topics, but still important to study scientifically! Our own system contains places potentially able to host life unlike on Earth. Not just Titan!
#AstroThread
Exotic solvents & life's building blocks are among the more speculative
#astrobiology topics, but still important to study scientifically! Our own system contains places potentially able to host life unlike on Earth. Not just Titan!
#AstroThread
https://twitter.com/People_Of_Space/status/1393504031209820161
All Earth life is carbon-based and needs water to survive. 💦
'Mildly' exotic life might share these traits, but use e.g. other information molecule (or differently coded DNA, even with different/more 'letters') or opposite chirality (left/right-handedness) of some compounds.

'Mildly' exotic life might share these traits, but use e.g. other information molecule (or differently coded DNA, even with different/more 'letters') or opposite chirality (left/right-handedness) of some compounds.

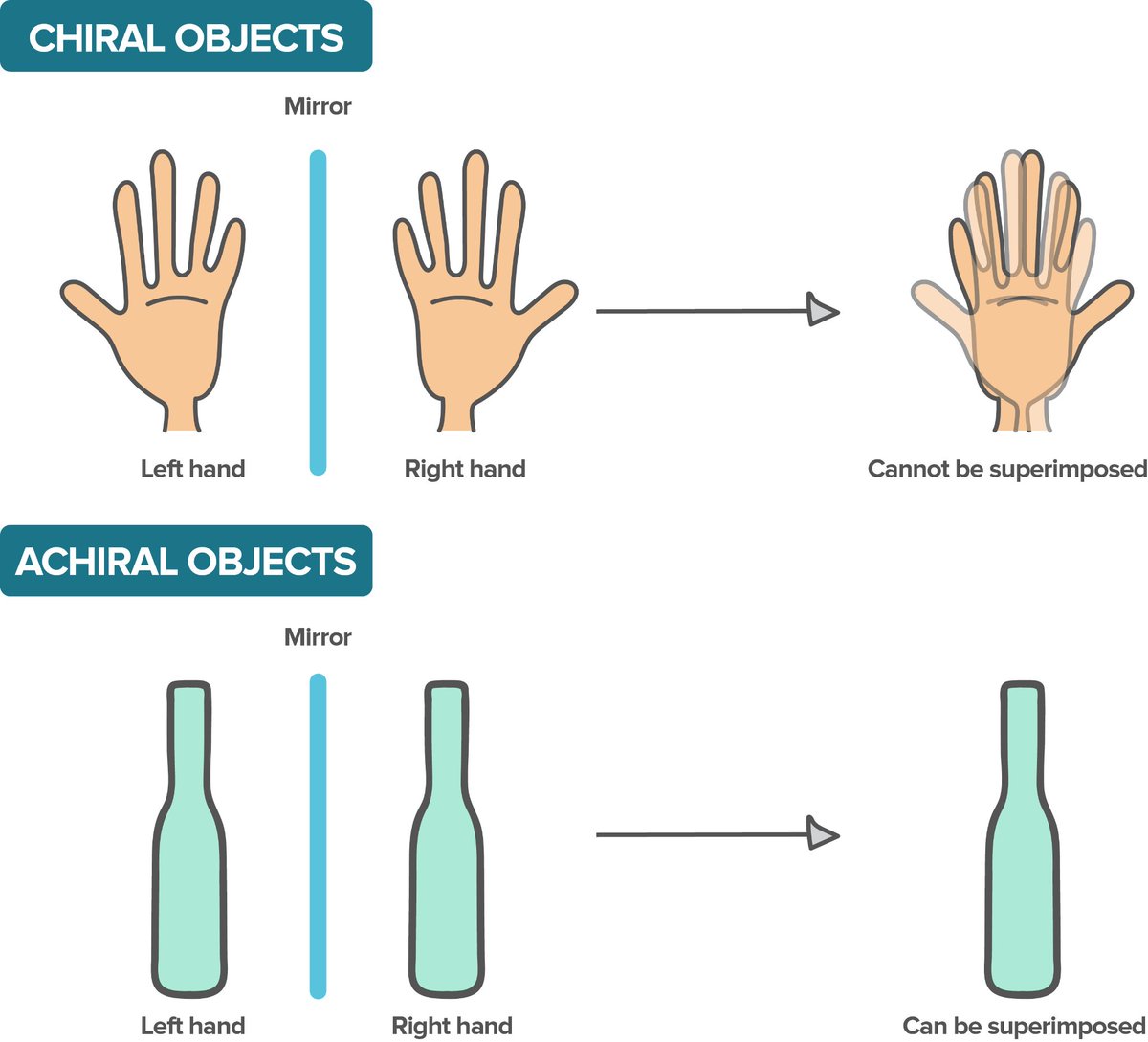
There are countless possibilities of different information molecules and their coding. Is Earth DNA and RNA a ', frozen accident', or does it have a phys/chem reason? And is all life chiral? In the same way, or is that another frozen accident? What about the amino acids we use? 

Discovery of extra-terrestrial life would help us answer those questions greatly. If we found life on Mars, Enceladus or elsewhere unrelated to Earth's, we'd know a great deal more!
So far these remain open questions, with great many proposed answers for us to test.
So far these remain open questions, with great many proposed answers for us to test.

That's all still carbon-based life in water, mind you! Other biochemistries might be possible, some quite similar.
You know hydrogen peroxide? Chemically aggressive, highly oxidizing; solutions used to bleach hair, as a surface disinfectant. Imagine *that* as a solvent for life!
You know hydrogen peroxide? Chemically aggressive, highly oxidizing; solutions used to bleach hair, as a surface disinfectant. Imagine *that* as a solvent for life!

We're back! Hydrogen peroxide: friend or foe? 🤔
If sufficiently diluted in water and in low temp (-> lower reactivity, less likely to break important building blocks 🧬), friend - possibly. Did you know it's hygroscopic - 'attracts' water? Low temp, dry... Guessed right: Mars!
If sufficiently diluted in water and in low temp (-> lower reactivity, less likely to break important building blocks 🧬), friend - possibly. Did you know it's hygroscopic - 'attracts' water? Low temp, dry... Guessed right: Mars!

A few papers hypothesizing that potential Martian life might use a mixture of water-hydrogen peroxide were produced by @extreme_microbe & colleagues. H2O2 also acts as an antifreeze - useful in low temp.
cambridge.org/core/journals/…
liebertpub.com/doi/abs/10.108…
lpi.usra.edu/meetings/absci…
cambridge.org/core/journals/…
liebertpub.com/doi/abs/10.108…
lpi.usra.edu/meetings/absci…
So far it's just a speculation that remains to be tested. Luckily there's no shortage of Mars missions! 🚀
💧Water can be mixed with other compounds, too. Sulfuric acid is highly aggressive, too - but also attracts water, and their mix is found in Venusian clouds.
💧Water can be mixed with other compounds, too. Sulfuric acid is highly aggressive, too - but also attracts water, and their mix is found in Venusian clouds.

Sulfuric acid-water mix as a solvent would be highly acidic, of course! Yet Earth life can thrive even in extremely acidic ponds, albeit inside the cells we find water (with all else needed for life in it).
But how stable long-term is the Venusian cloud cover? We don't know! 🤷♀️
But how stable long-term is the Venusian cloud cover? We don't know! 🤷♀️

I'm so sorry to say that I'm feeling ill :(. I hope it's not infectious, let alone covid. If I'm unable to continue this thread here before the new host starts, I'll finish it at @Julianne_SF as soon as I can. Sorry again :(.
Good news everyone! 👋 Since there fortunately was a slot open for this week, I opted to continue the thread here.
Let's have a look at more water-other solvent mixtures today 💦, and head to the territory of really exotic solvents and building blocks for life tomorrow!
Let's have a look at more water-other solvent mixtures today 💦, and head to the territory of really exotic solvents and building blocks for life tomorrow!
Last weekend, we had a tiny look at non-DNA/RNA information molecules and chirality, and mixtures of water with hydrogen peroxide or sulfuric acid as solvents for potential alien life.
Could water be mixed with something else? Indeed could, likely often is! Enter NH3: ammonia...

Could water be mixed with something else? Indeed could, likely often is! Enter NH3: ammonia...


Ammonia is abundant in the outer solar system, approx. from Saturn further on.
Besides its amazing surface chemistry, which we'll probe tomorrow, 🪐 moon Titan likely has a subsurface ocean of water mixed with a little ammonia.
Could there possibly be life in such a place?
Besides its amazing surface chemistry, which we'll probe tomorrow, 🪐 moon Titan likely has a subsurface ocean of water mixed with a little ammonia.
Could there possibly be life in such a place?

Like water, ammonia is a polar solvent & behaves similarly in many ways. It's alkaline, which would change some properties of life. Its behavior with water, and behavior of their ices, is quite complex! This is a nice paper on it, but quite technical:
sciencedirect.com/science/articl…
sciencedirect.com/science/articl…
With slight amounts of ammonia, life might work similarly to Earth. With its growing concentration, the biochemistry would change. Oxygen-containing carbonyl/hydroxyl groups might be replaced by nitrogen-containing groups.
Still, not that far from Earth life. Speaking of far...
Still, not that far from Earth life. Speaking of far...

Ammonia works as an antifreeze for water. With ammonia and salts, water under thick ice could stay liquid long even on bodies with meager heat sources, such as dwarf planets in the Kuiper Belt and beyond like Pluto or Eris - even so faraway, small and eccentric ones like Sedna! 

Sedna is a favorite of mine; after all, I'd set a story in it because it fascinated me so.
clarkesworldmagazine.com/novakova_10_13/
clarkesworldmagazine.com/novakova_10_13/
Aside from stories, there are scientific papers modeling the potential for subsurface oceans in the outer solar system - which ammonia would greatly contribute to. Here's a staple paper on the topic by Hussmann et al.:
sciencedirect.com/science/articl…
sciencedirect.com/science/articl…
Any potential life in such cold, ammonia-laden, briny oceans would be very different from ours, having to persist on meager resources, and unfeasible for us to discover in the foreseeable future. Its existence seems unlikely... but not entirely impossible.
One day, we might see.
One day, we might see.

That was it for the main water/other solvent mixtures. Tomorrow, we'll head into a far more exotic territory, but fear not - we'll still have published scientific work to guide us there!
We'll go for instance where the solvent is not polar, but the temperature kind of is...
We'll go for instance where the solvent is not polar, but the temperature kind of is...
Exotic solvents: Could something entirely replace the role of water for some alien life? And is a liquid solvent necessary at all?
These are very hypothetical questions, but should be addressed by science if we're to have an inkling what to look for!
These are very hypothetical questions, but should be addressed by science if we're to have an inkling what to look for!

While life in a solid or thin gaseous medium has been considered, it seems unlikely at best; a liquid can dissolve and distribute compounds needed for life, transport them between reaction sites... But a supercritical fluid ('between liquid and gas') could work very well too. 
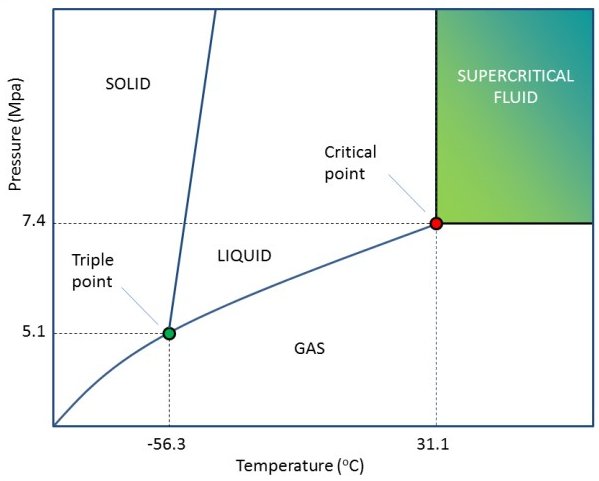
We'll stay with liquids (+ supercritical fluids) then. Preferably something abundant. Water in some state is EVERYWHERE we look in the universe.
While lots of exotic solvents were proposed, we might have trouble finding some in pure form in big amounts.
I mean: hydrazine ocean?
While lots of exotic solvents were proposed, we might have trouble finding some in pure form in big amounts.
I mean: hydrazine ocean?

Not kidding here; hydrazine really has been considered! We know it as rocket fuel 🚀, but its dipole moment and viscosity are similar to water, temperature range under standard pressure large. It's very reactive, though, so not an ideal candidate unless in cold... ❄️
Liquids like these, which are probably rarely found in large quantities and seem to have other more or less serious drawbacks, have been considered as solvents for life mostly as a mind exercise and in order not to omit interesting options, but don't seem very likely. 

They include even compounds likely very important for prebiotic reactions, such as formamide - but how likely is a formamide ocean, even a pond? 🌊
It's not entirely impossible, but let's focus on liquids more readily found in the universe.
It's not entirely impossible, but let's focus on liquids more readily found in the universe.

Ammonia is one. We've already looked at its mixture with water. Lakes or oceans of relatively pure ammonia could potentially exist on planets or moons in cold regions of their star systems, under high pressure (a thick atmosphere) - but how to get rid of the water? 

If it's very cold, water would be just another rock - and we know a world on whose surface water ice acts as rock and other compounds exist as liquids. Not ammonia, though. It's light hydrocarbons, like methane and ethane, and the world is Saturn's moon Titan. 🪐 

Titan is the only moon in our system with a substantial atmosphere, and in many ways resembles Earth: It's cloudy, it rains, it has deserts, lakes, seas... But what cycles there isn't water, for it's far too cold for it; it's methane and ethane.
Could it possibly support life?
Could it possibly support life?

Light hydrocarbons like methane and ethane are non-polar, unlike water; their charge is evenly distributed.
That's greatly important for life! It influences what can be dissolved and how a cellular membrane would work. This would be truly exotic life!
That's greatly important for life! It influences what can be dissolved and how a cellular membrane would work. This would be truly exotic life!

So how would life on Titan work?
Earth life cells exist within double-layered membranes. Each layer turns its 'water-loving' side outside/inside the cell, where water is, and the 'water-repulsive' side within the membrane.
In a non-polar solvent, we'd need the exact opposite.
Earth life cells exist within double-layered membranes. Each layer turns its 'water-loving' side outside/inside the cell, where water is, and the 'water-repulsive' side within the membrane.
In a non-polar solvent, we'd need the exact opposite.

We couldn't just reverse Earth cell membrane, though. The fats it's composed of would turn too rigid in the freezing -180 °C on Titan. Nowhere on Earth it gets anywhere near this cold! 🥶
We would need something new - and that's where chemistry and computer modeling come in!
We would need something new - and that's where chemistry and computer modeling come in!

Scientists looked at what compounds would stay fluid enough on Titan and what the membrane would look like: Enter the azotosome (simplified on the right image)! Nitriles were found to be the best membrane candidates for Titan. 🦠
advances.sciencemag.org/content/1/1/e1…
advances.sciencemag.org/content/1/1/e1…

Vinyl cyanide, a good candidate, was even detected on Titan using the ALMA array.
Does it mean life, though? No! It just means a greater chance of it. But could cell membranes really assemble on Titan like bi-layered lipid membranes do on Earth?
advances.sciencemag.org/content/3/7/e1…
Does it mean life, though? No! It just means a greater chance of it. But could cell membranes really assemble on Titan like bi-layered lipid membranes do on Earth?
advances.sciencemag.org/content/3/7/e1…

A recent paper suggests that self-forming of cell membranes on Titan is unlikely - but proposes an even more exotic option: Life without any membranes! Is *that* even possible?
advances.sciencemag.org/content/6/4/ea…
advances.sciencemag.org/content/6/4/ea…
The authors argue that membranes are unnecessary on Titan, since there's a much lower chance of harmful reactions in such cold, and no need for a protective membrane.
They obviously don't come from biology!
Look at this simplified cell representation and tell me what you see...
They obviously don't come from biology!
Look at this simplified cell representation and tell me what you see...

I see membranes everywhere. Eukaryotic cells have organelles, and almost everything important occurs on their membranes. They hold chains of enzymes, bring compounds together... Bacteria and archea are small and lack organelles, but things go about mostly on their cell membranes. 

I wouldn't say membranes are strictly necessary (the universe is big), but I'd say life with membranes is far more likely - even on Titan.
How would life there get its energy, though?
Metabolizing hydrogen and producing methane (then usable by other life) is one suggested way.
How would life there get its energy, though?
Metabolizing hydrogen and producing methane (then usable by other life) is one suggested way.

The atmospheric chemistry of Titan doesn't exclude that; there's even a surprisingly low amount of hydrogen near the surface... Still, it's nowhere near evidence of life.
We need to wait for missions to explore Titan right there, starting with Dragonfly (to be launched in 2027).
We need to wait for missions to explore Titan right there, starting with Dragonfly (to be launched in 2027).

For more on chances of life on Titan, this is a good, quite accessible article to start with.
But we need to move on now, to other solvents and beyond Titan...
mdpi.com/2075-1729/6/1/…
But we need to move on now, to other solvents and beyond Titan...
mdpi.com/2075-1729/6/1/…
If you thought Titan was cold, wait until we dive into seas of liquid nitrogen and leave carbon-based life's chemistry behind!
We'll visit Pluto-like worlds now, with the important difference that on Pluto, nitrogen is solid. How do we get a liquid sea of it?
We'll visit Pluto-like worlds now, with the important difference that on Pluto, nitrogen is solid. How do we get a liquid sea of it?

The answer is pressure. Pluto has a tenuous atmosphere, and we need the right combo of temperature and pressure to get the phase we want - liquid in this case.
Under 1 Earth atmosphere, nitrogen is liquid just between -210 and -195 °C. Not a great big range, right?
Under 1 Earth atmosphere, nitrogen is liquid just between -210 and -195 °C. Not a great big range, right?

However, increase the pressure about tenfold (still much less than atmospheric pressure on Venus ;)), and the range is almost twice as large and has moved to higher temperatures.
We could imagine a dense-atmosphere super-Earth far away from its sun, or even free-floating...
We could imagine a dense-atmosphere super-Earth far away from its sun, or even free-floating...

Such an ocean would behave very differently than water oceans on Earth, mind you! For one thing, nitrogen ice is heavier than liquid & would sink. Water is quite unique with its crystalline ice that has a lower density than liquid, floats on it and shields it from total freezing. 

That might be a problem if our planet cools down... but thick atmospheres are good for keeping heat locked in + a large planet would have lots of radiogenic heat.
But what life would be able to exist in liquid nitrogen?
We need to move from carbon to another element: silicon.
But what life would be able to exist in liquid nitrogen?
We need to move from carbon to another element: silicon.

Sci-fi is rife with silicon monsters, from Horta to the 'crystalline entity', and even HG Wells was dreaming of silicon life!
Most get one major thing wrong: we don't want heat and we don't want 'stone' - silicates. They're the most usual form of silicon on Earth...

Most get one major thing wrong: we don't want heat and we don't want 'stone' - silicates. They're the most usual form of silicon on Earth...


...but in biology, silicates are of little interest (geologists forgive me). They're very stable, non-reactive, rigid. We can melt them, alright, but they don't do anything interesting from the point of view of biology even then.
Sorry. No lava monsters today.
Sorry. No lava monsters today.

Silicon does get interesting, though, in cold, without water and oxygen. Then it can form silanes, which have a great variety of forms, side chains, chiral variants... They could possibly form functional analogs to carbon-based biomolecules. 
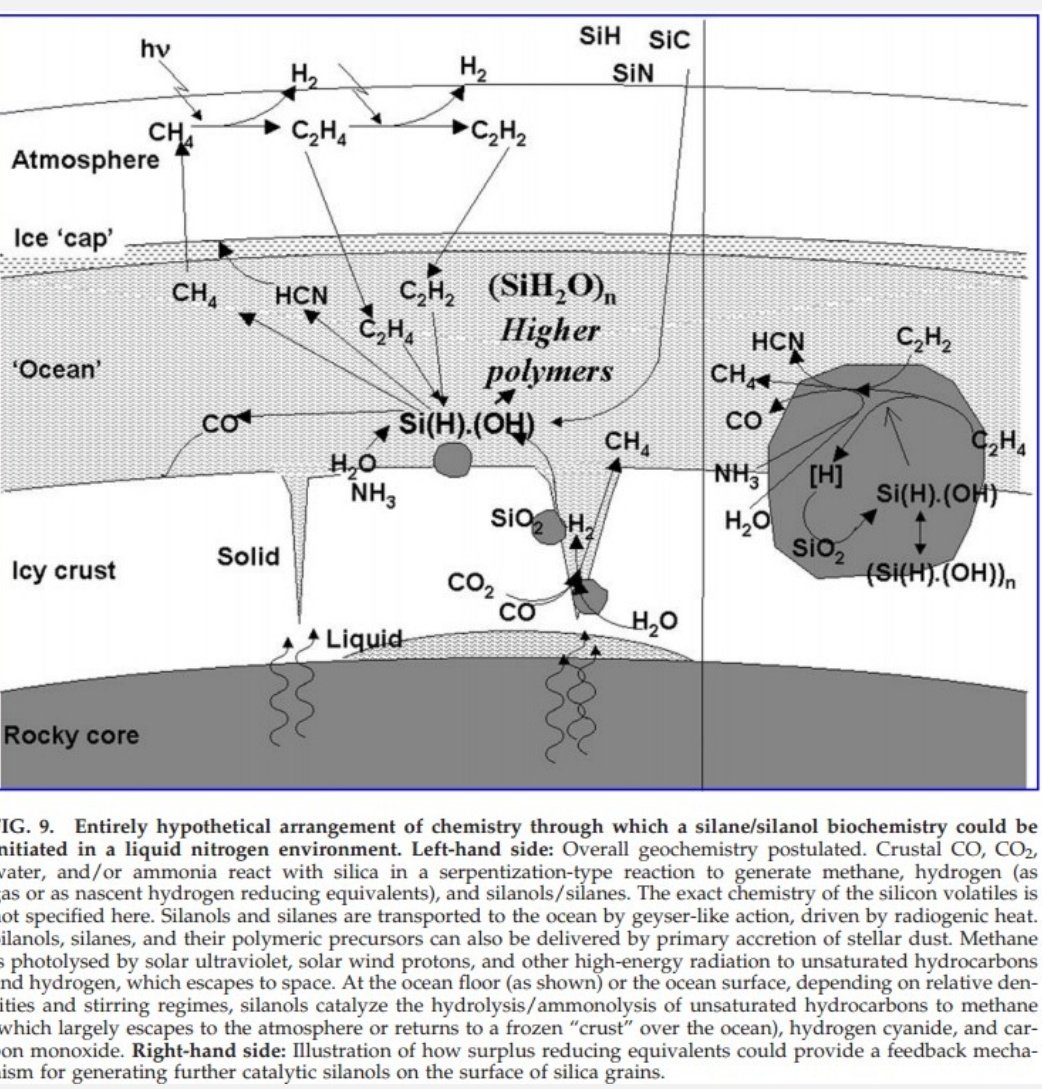
'Cryosolvents' such as liquid nitrogen, but possibly even argon or neon (which are much rarer than nitrogen, though) would be ideal for silane-derived biochemistry.
Polysilanes are also photo-conductors: could that work as an exotic energy source? Questions everywhere!
Polysilanes are also photo-conductors: could that work as an exotic energy source? Questions everywhere!
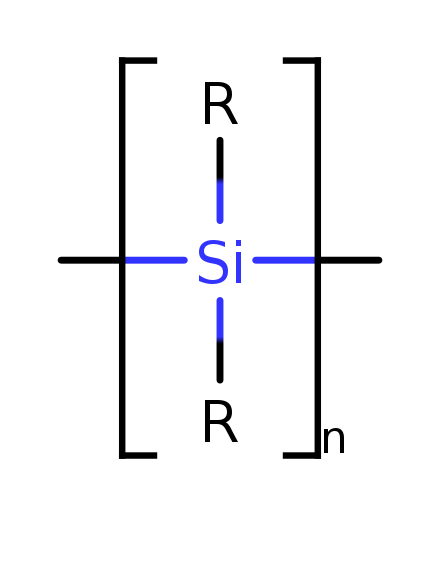
It's going to take time to find the answers. Silicon biochemistry in cryosolvents will likely long remain hypothetical. Planets that could support it won't be prime (or easy) spectroscopy targets and we don't know what chemical traces of life we should look for on them. 

In theory, there could be short-lived capsules of liquid nitrogen on Triton, Pluto etc, but liquid nitrogen wouldn't stably exist under its ice, which is denser. Not much chance of exotic life.
One day, when the Sun is at its brightest, Pluto might briefly have a nitrogen ocean.
One day, when the Sun is at its brightest, Pluto might briefly have a nitrogen ocean.

Since we've started the topic of exotic life's backbones: anything else then silicon?
Nitrogen, boron, phosphorus, sulphur and their combinations were proposed; none seem as hopeful as silicon, however, and silicon seems at a great disadvantage to carbon.
Nitrogen, boron, phosphorus, sulphur and their combinations were proposed; none seem as hopeful as silicon, however, and silicon seems at a great disadvantage to carbon.

Abundance is an advantage, and so is chemical versatility and likely the ability to share pi electrons in aromatic compounds.
Like water seems to be the most abundant solvent, carbon is the most abundant backbone element.
Like water seems to be the most abundant solvent, carbon is the most abundant backbone element.
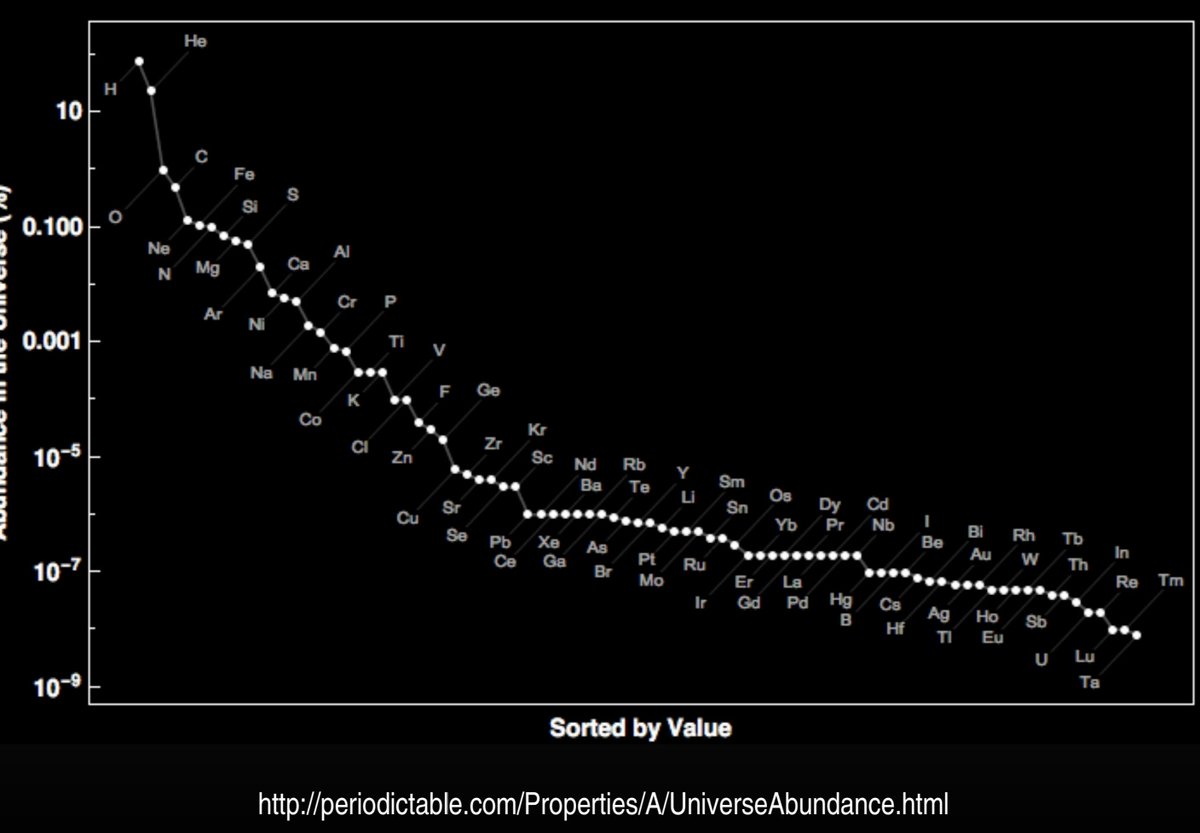
Personally, I'd bet on most life in the universe using the water-carbon combo, with mixed solvents like water-ammonia or light hydrocarbons (still with carbon life) as the most likely other possibilities. Silicon biochemistry would be second to carbon, but much rarer. 

There are still a handful of intriguing options we haven't explored here yet; you can look forward to these tomorrow! 🚀
A hint: Io-like worlds; various types of rogue planets; seas of gas & ice giants; brown dwarfs... See you tomorrow, exploring the universe together! 🌌
A hint: Io-like worlds; various types of rogue planets; seas of gas & ice giants; brown dwarfs... See you tomorrow, exploring the universe together! 🌌

We're back with exotic life! We've looked at some potential alternative solvents & building blocks, but let's explore a few very exotic environments that might hypothetically host life. We'll start with Io-like worlds!
'Io?!' you may exclaim. 'That water-devoid volcanic hell?'
'Io?!' you may exclaim. 'That water-devoid volcanic hell?'

Io is being so squeezed by tidal forces that it's lost its volatiles (easily evaporated substances, like water) long ago. It's an ever-changing world of hundreds of active volcanoes.
How could any world like that support life?
Let's look just beneath the surface: at lava tubes.
How could any world like that support life?
Let's look just beneath the surface: at lava tubes.

Lava tubes can be found basically anywhere with rock-based volcanism. They could be a favorable life site on Mars. Useful for settling the Moon and Mars.
Their inside is protected from radiation, could have relatively stable conditions, retain moisture...
'But on Io?!' you ask.
Their inside is protected from radiation, could have relatively stable conditions, retain moisture...
'But on Io?!' you ask.

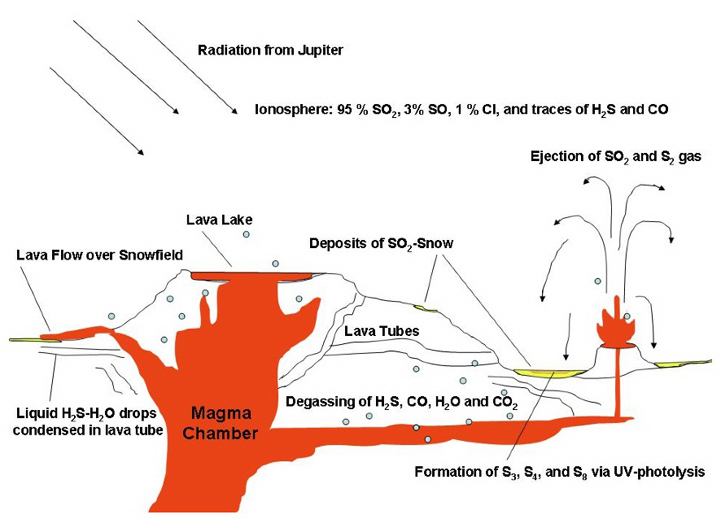
Admittedly, it's unlikely, but merits a scientific look. Could Io have retained a little water, which could stay liquid underground? Or could there be a sulfur-based solvent for life? SO2 could be liquid under Io-like subsurface conditions... Energy sources wouldn't be the issue. 

.@extreme_microbe briefly looked at the chances of life on Io: journalofcosmology.com/SearchForLife1…
Could there be sulfur-based life in a solvent like sulfur dioxide, hydrogen sulfide or sulfuric acid? Unlikely; but it could be a mistake to dismiss Io automatically, without consideration.
Could there be sulfur-based life in a solvent like sulfur dioxide, hydrogen sulfide or sulfuric acid? Unlikely; but it could be a mistake to dismiss Io automatically, without consideration.
One note re the article linked in the previous tweet: While this paper is scientifically sound, if speculative, and I have no qualms linking it, the journal where it had appeared is definitely not a good one. Lots of - forgive the term - batshit papers appeared there over time.
There's a downside to astrobiology: it attracts all sorts of outrageous claims. 'Animals spotted on Venus! Fungi are seen growing on Mars!' (Usually it's this sort of wishful thinking and misinterpreting images from probes. A bit like Lowell believing in artificial Mars canals.)
Speculating on exotic Io life is one thing; can be done rigorously, even if we have little data. To claim 'life exists on Io' would be another, unscientific.
Back to the theme: A new paper explores the chances of life in sulfuric acid, finding them slim.
mdpi.com/2075-1729/11/5…
Back to the theme: A new paper explores the chances of life in sulfuric acid, finding them slim.
mdpi.com/2075-1729/11/5…
There may be lots of Io-like worlds over the galaxy: close-in planets with lava-oceans on their daysides, tidally squeezed worlds in packed exoplanet systems... While the likelihood of life on them seems very, very slim, it's interesting to consider it for future reference. 

What else is there 'lots of'? Rogue planets!
According to simulations, their number may exceed the no. of stars in the galaxy by an order of magnitude. Most probably kicked out of their systems by the early gravitational dance of planets, they wander... and may do so with life.
According to simulations, their number may exceed the no. of stars in the galaxy by an order of magnitude. Most probably kicked out of their systems by the early gravitational dance of planets, they wander... and may do so with life.

~25 suspected rogues have been spotted by gravitational lensing. Some may be low-mass brown dwarfs (we'll get to them too!) rather than planets.
Their traits are poorly constrained + we can't repeat the chance observations. But in overall, rogue planets could come in all sorts!
Their traits are poorly constrained + we can't repeat the chance observations. But in overall, rogue planets could come in all sorts!

Lower-mass rogues could resemble Ganymede or Callisto. With no star or tides to heat them, their surfaces would be frozen. But under thick ice crusts, they could retain liquid water or water-ammonia oceans for billions of years, if they had enough radiogenic heat. 

Alas, we'd never know if they had life - no chance of observing its traces at a distance. But it's a great environment for SF writers ;).
Other rogues could be more massive: have more inner heat and retain a thick greenhouse atmosphere. Thick enough to keep surface liquid water!
Other rogues could be more massive: have more inner heat and retain a thick greenhouse atmosphere. Thick enough to keep surface liquid water!

David J. Stevenson speculated so already in 1999 in @Nature: nature.com/articles/21811
Early atmospheres contain lots of hydrogen from the star- & planet-forming disk. Planets 'kicked out' early enough may hold onto a lot of it, and hydrogen is a very efficient greenhouse gas!
Early atmospheres contain lots of hydrogen from the star- & planet-forming disk. Planets 'kicked out' early enough may hold onto a lot of it, and hydrogen is a very efficient greenhouse gas!
Indeed, such H2-greenhouse planets may be abundant not only as rogues, but also on star system peripheries.
Life could gain energy by making ammonia out of hydrogen and nitrogen - but wouldn't it destroy its own greenhouse in time, unless the ammonia broke down fast enough?
Life could gain energy by making ammonia out of hydrogen and nitrogen - but wouldn't it destroy its own greenhouse in time, unless the ammonia broke down fast enough?

Ammonia and other gases could be recognizable biosignatures on such H2-worlds - which is great, because we could detect signs of life at a distance, possibly even on a rogue planet!
iopscience.iop.org/article/10.108…
iopscience.iop.org/article/10.108…
Finally, rogues could be massive planets with a system of moons - which could be tidally heated and resemble moons of Jupiter or Saturn.
Even pairs like Earth-Moon could survive ejection from their systems - and potentially retain habitability: iopscience.iop.org/article/10.108…
Even pairs like Earth-Moon could survive ejection from their systems - and potentially retain habitability: iopscience.iop.org/article/10.108…
Numerous across the galaxy are also brown dwarfs: objects that are not-quite stars, but different from planets. Much more massive than Jupiter, they can be similar size or even smaller due to being more gravitationally compressed. They come in various masses, temperatures... 

Some could have habitable moons/planets (whatever you'd call it), but the more exotic option is life directly on brown dwarfs. Some of the low-temp have water ice clouds. Could there be an 'atmospheric habitable zone' for them?
iopscience.iop.org/article/10.384…
iopscience.iop.org/article/10.384…
More papers emerged (including by Avi Loeb, who writes on anything/everything): iopscience.iop.org/article/10.384…
An earlier one even considers the possibility of life in exotic solvents on sub-brown dwarfs: sciencedirect.com/science/articl…
It's a very exotic option - but potentially detectable.
An earlier one even considers the possibility of life in exotic solvents on sub-brown dwarfs: sciencedirect.com/science/articl…
It's a very exotic option - but potentially detectable.
The possibility to test a scientific hypothesis is at the core of science. What's currently untestable may be a nice speculation, but would remain just that for a long time.
But we can *look* at the spectra of low-mass brown dwarfs, H2-worlds etc. to search for biosignatures.
But we can *look* at the spectra of low-mass brown dwarfs, H2-worlds etc. to search for biosignatures.

Finally, habitable planets could exist around stars that have completed their time on the main sequence and gone through a cataclysmic 'end'.
White dwarf planets may actually be a good site to search for life. Neutron stars' planets? More speculative...
clarkesworldmagazine.com/novakova_02_18…
White dwarf planets may actually be a good site to search for life. Neutron stars' planets? More speculative...
clarkesworldmagazine.com/novakova_02_18…
I'd love to cover even more of the 'exotic life' topic (seas of supercritical hydrogen; potential strange reactions on neutron stars...), but we could go on & on without really exhausting the possibilities.
I've covered the most likely/frequent. Time to wrap up with a few links.
I've covered the most likely/frequent. Time to wrap up with a few links.

A great overview of potential alternative solvents, some building blocks and more or less exotic energy sources can be found in this very accessible textbook:
springer.com/gp/book/978354…
springer.com/gp/book/978354…

For a shorter take, this is a great paper to start with: sciencedirect.com/science/articl…
This study report is also good to start with (the pdf is free): nap.edu/catalog/11919/…
We may find that exotic life is extremely rare; we may only keep discovering carbon-based life in water, if any. Some exotic environments may remain forever unreachable for our exploration efforts. But some, like Titan, are right in our backyard.
Let's keep exploring, then. 🔭
Let's keep exploring, then. 🔭

• • •
Missing some Tweet in this thread? You can try to
force a refresh