I'll try to interpret the *data* I've presented in this context:
The Indian approach cab be best characterized as opportunistic. It considers that certain things can be affected and certain things cannot. It attempts to maximize what can be leveraged. Let us explore:
1/
The Indian approach cab be best characterized as opportunistic. It considers that certain things can be affected and certain things cannot. It attempts to maximize what can be leveraged. Let us explore:
1/
https://twitter.com/prasannavishy/status/1394193044866359299
Given the choice to develop N vaccine candidates (N can be few or many), certain invariants hold:
* Each of N takes 3 steps to approval.
* Each will take a minimum time to clear all 3.
* Addi resources or options will not speed this up ('9 women and a baby in 1 month..')
2/
* Each of N takes 3 steps to approval.
* Each will take a minimum time to clear all 3.
* Addi resources or options will not speed this up ('9 women and a baby in 1 month..')
2/
Therefore during the discovery phase the govt chose to be an enabler. As Poonawalla's tweets in Apr-Dec 2020 showed, Govt facilitated their scaling up and encouraged them to sign big deals, and start production early.
3/
3/
Back in Nov, realizing that Covaxin was likely to succeed (it cleared Ph 1/2 in early Dec) it lined up investments in @BharatBiotech expansion, and spend money on BSL-3 line upgradation at two PSUs (IIL and BIBCOL) and now Haffkine and Biotec Pune.
4/
4/
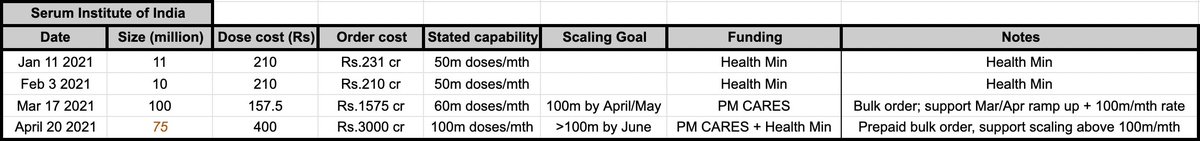
The result is that 3 months post AZ1222 (Covisheild) Ph3, SII had produced over 200m doses, while Pfizer+Moderna combined had yet to get to that figure - they both reached 100m doses around 3mo and a few weeks after their respective Ph3 and EUA.
5/
5/
Covaxin Ph3 (early Mar) aligned more closely with J&J (end Jan). Yet, J&J hit catastrophic production problems with all its ~80m production in US to date discarded. Its 24m production is all in Netherlands.
6/
6/
Covaxin much outstripped J&J in production volume, and will exceed Moderna prodn rate by late summer.
The opportunistic element here is that given that regardless of input investment, only a few will succeed early.
7/
The opportunistic element here is that given that regardless of input investment, only a few will succeed early.
7/
This is true regardless of whether one writes blank cheques (US) or not (India). Some will fail outright, some will take much longer to clear 3 steps. This isn't 'failure' - it's the nature of drug discovery.
8/
8/
It is possible to construct a mathematical model of this (but I'd defer to legends like @agrawalmanindra and @stellensatz) that expresses this as mathematical construct: that given these constraints, what is the best point to put your money in to maximize production.
9/
9/
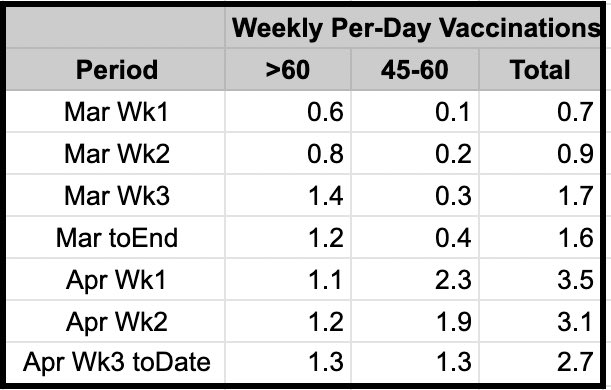
However that's essentially what India did - supported each as necessary, then helped push production rate up as quickly from approval date as possible. Rampup-from-approval view would show Indian production scaled up faster than Pfizer, Moderna, JJ.
10/
10/
To be fair there are scaling concerns for SII because 100-150m doses from a single plant is hard for anyone. They need the extra line and they've copped delays there. Covaxin on the other hand has multiple lines coming up - an early lesson from SII they learned.
11/11
11/11
• • •
Missing some Tweet in this thread? You can try to
force a refresh