There’s a lot of recent handwaving about Covaxin focusing on sensationalism around ‘lack of WHO EUL’. The status of this information can be readily found:
extranet.who.int/pqweb/sites/de…
@BharatBiotech submitted the EOI only a month ago, with pre-submission meeting in May/June.
1/
extranet.who.int/pqweb/sites/de…
@BharatBiotech submitted the EOI only a month ago, with pre-submission meeting in May/June.
1/

The WHO guidelines are listed here: who.int/publications/m…
This qualifies everything from the trials process to manufacturing.
2/
This qualifies everything from the trials process to manufacturing.
2/
An interesting example here is J&J, which received an ok of its US/Netherlands manufacturing sites in March:
But then the entire US production has been under question. All US J&J vaccines used are imported from Netherlands.
news.yahoo.com/100-million-do…
3/
But then the entire US production has been under question. All US J&J vaccines used are imported from Netherlands.
news.yahoo.com/100-million-do…
3/

Back to Covaxin and its WHO EUL process, essentially all approved vaccines are from entities with the money to run trials in multiple places. A summary:
covid19.trackvaccines.org/vaccines/
The number of approvals and trials are a function of monetary and geopolitical power.
4/
covid19.trackvaccines.org/vaccines/
The number of approvals and trials are a function of monetary and geopolitical power.
4/
Covaxin has been already produced in more volume than J&J and Sputnik. The cumulative 20m J&J vaccines imported in US from J&J’s EU site is less than May’21 Covaxin output. Given production ramp up, well over 0.5 billion doses of Covaxin will be consumed by end 2021.
5/
5/
The sensationalism of this topic is pointless. It’s worth noting that Sinovac and Sputnik V are not WHO EUL listed. J&J is listed despite its main production site being verified and yet everything ever produced there (the controversial Emergent Biosolutions) being spoiled.
6/
6/
Covaxin will over the next few months have the volume to be probably the most consequent COVAX candidate. It’s much cheaper than mRNA options. Even Sinopharm goes for Rs.2600 a dose:
7/
nytimes.com/2021/03/12/wor…
7/
nytimes.com/2021/03/12/wor…
A WHO EUL means 2 things - a clearance standard and the ability to supply worldwide Gavi/COVAX supply pipeline to developing world.
1. Acceptance of a vaccine for travel purposes. The Biden Administration is opposed to vaccine passports:
voanews.com/episode/why-us…
8/
1. Acceptance of a vaccine for travel purposes. The Biden Administration is opposed to vaccine passports:
voanews.com/episode/why-us…
8/
Regardless of whether a vaccine has WHO EUL, many do not want to have to prove whether they have been vaccinated at all. Until vaccine passport system is standardized, whether or not a vaccine is in EUL is irrelevant. It means nothing today.
9/
9/
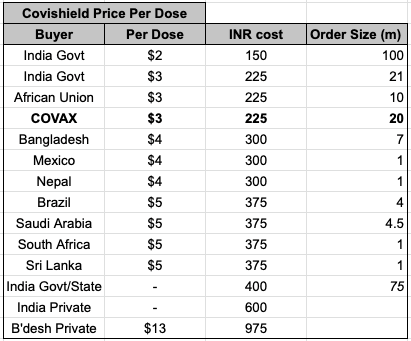
COVAX supply is arguably a far more important consequence. Covaxin at under $15 a dose to international supply (with additional subsidization from WHO) will undercut essentially every other vaccine - even Covishield which it can exceed in production volume by end 2021.
10/
10/
@BharatBiotech ’s WHO EUL application timeline is well aligned to its production ramp up, and India currently will not support exports of its vaccine production anyway. They will have their EUL much before popular support for exporting their production in volume.
11/11
11/11
• • •
Missing some Tweet in this thread? You can try to
force a refresh