
The story of how @Altris_ab came to be and my involvement in PBA research is also a nice one. It was really a combination of the right people meeting at the right time and at the right place.
It began with Ronnie Mogensen, who was working on polymer electrolytes at the time and just needed a reliable positive electrode that was easy to make. He tried NaFePO4 which didn't always function. Then he turned to NaxFe[Fe(CN)6]. This always worked to a reasonable degree
He then stumbled upon a paper where the group of John Goodenough (Nobel laureate in chemistry for Li-ion #batteries) made rhombohedral Prussian white. pubs.acs.org/doi/10.1021/ja…
What is Prussian white? Specifically, when the sodium content in NaxFe[Fe(CN)6] reaches a maximum with few structural defects, the material adopts a white colour rather than its famous blue. 

It also has very impressive electrochemical performance with a capacity around 160 mAhg-1 and an average voltage of 3.4 V. Needless to say, @RezaYounesi was intrigued and wanted to produce the material for his #sodium #battery research.
The problem was the synthesis, which required the use of a hydrothermal reactor with a built in stirrer. We did not have such an autoclave available so he needed an alternative approach.
It should be mentioned that until this stage, most synthesis routes to prepare PBAs for electrodes resulted in low capacities and low reversibility due to the presence of [Fe(CN)6]3- vacancies and water in the structure. So this needed to be addressed
This was around the time that I got involved in the project, purely because I happened to be in the department and eager to collaborate. However, I had some light experience working with PBAs and methods of chemically intercalating materials. So together we devised a new approach
The original idea of the synthesis was to optimise for low vacancies, high sodium content and low water in separate steps with sodium and water contents reliant on a low vacancy content. We split up the process and recreated the result from Goodenough but with simple equipment. 

Given our success Reza suggested we should patent this, why not? Afterall, in Sweden the researchers own the idea, not the university. Of course we needed to pay for this ourselves but thanks to the generousity of @KristinaEdstrm2 we had available funding to do just that.
So we were pretty happy with managing to patent our idea. However, it was not the end as another chance encounter with Adam Dahlquist from @InnoEnergyEU pushed us in a new direction: Creating @Altris_ab
Adam introduced us to Paul Larsson to take care of the business side of things and we set to work on first creating the company and then taking on the hard work to get into the EIT InnoEnergy Highway® program, which in 2017 we succeeded in doing
This was quite unusual because when we started the application, Altris didn't even exist. But the four of us really believed in the idea. Plus, the willingness to bring in Paul and share the company was positively recieved. Afterall 1% of 1 million is better than 100% of nothing
Now the real work began. Scaling up production. Here we hired Dr Tim Nordh as our first ever employee and began to scale up the synthesis within the @StructuralChem1 labs. 
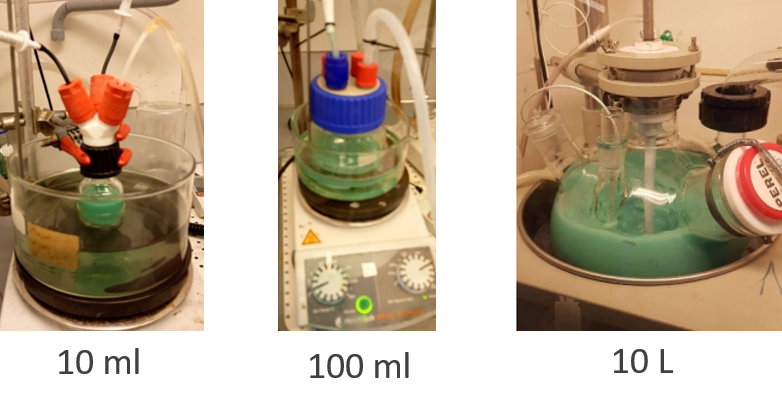
We also started collaborating with a local company LiFeSiZE to produce prototype #batteries. 



These were extremely challenging years as we were finding our feet as a company, looking for financing, trying to get things right with scaling up the synthesis AND building prototype cells for the first time. Many road blocks were encountered.
But we persevered! And very soon out grew the university as we were ready for the next step. Adam Dahlquist joined the company in 2019 and began pushing expansion. We obtained a site for a small pilot factory and then built the place ourselves (or Tim and Ronnie did).
We had a new challenge to achieve now. Cell producer partners wanted our Prussian white (which we trademarked as "Fennac" and they wanted a lot (or a lot by our standards). 50 kg by 2020 which was 50 times more than we had ever produced before. 

Of course, then Corona hit. But this didn't slow us down. Altris had won funding from @vinnovase and @Energi_mynd and came out over subscribed in the seed round of investments.
Since then, things have been ever accelerating and now @Altris_ab is working with cell producers in China, India and Europe to bring Fennac based batteries to market.
• • •
Missing some Tweet in this thread? You can try to
force a refresh























