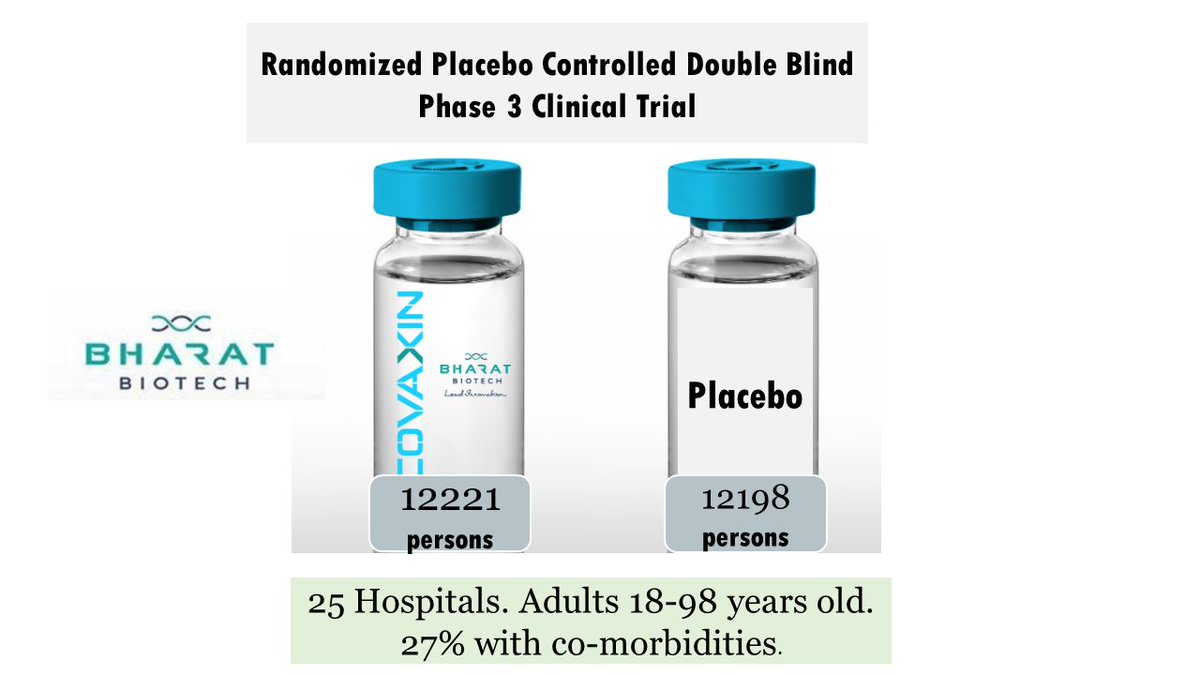
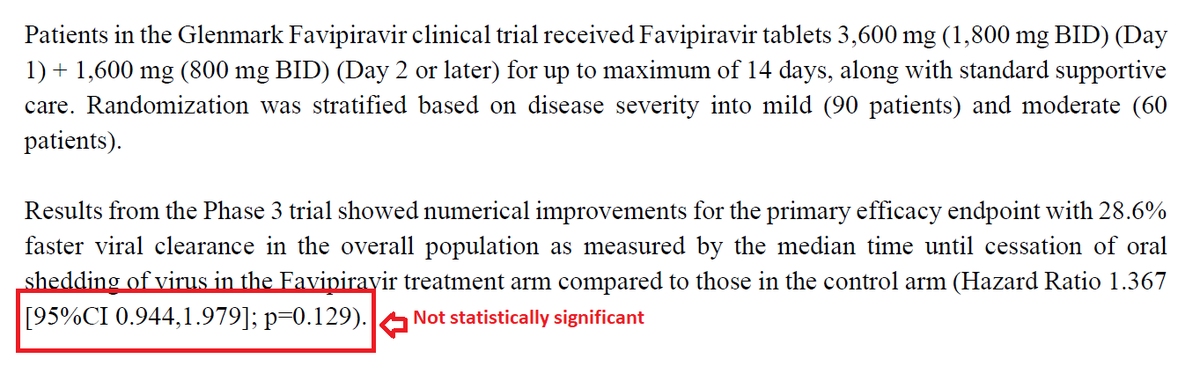
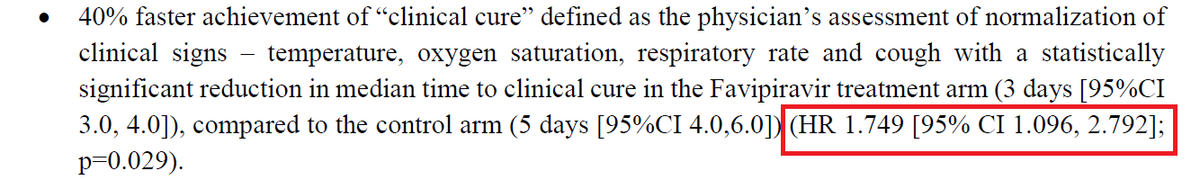A long thread on snakebite- a neglected public health problem in India.
1/N
India has the highest number of deaths due to #snakebites in the world. Snakes bite, injure, or disable hundreds of thousands of people and kill close to 58 000 Indians each year.
1/N
India has the highest number of deaths due to #snakebites in the world. Snakes bite, injure, or disable hundreds of thousands of people and kill close to 58 000 Indians each year.

2/N
Victims of snakebite often need ICU admission and access to ventilators and dialysis. Venomous snakebite is more expensive to treat than treating a heart attack or a stroke.
Victims of snakebite often need ICU admission and access to ventilators and dialysis. Venomous snakebite is more expensive to treat than treating a heart attack or a stroke.
3/N
People living in villages are at risk of snakebite because their housing conditions are poor and inadequate lighting makes it easy for the snakes to access their living spaces. Open-style habitation, open defecation and the sleeping on the floor often expose people to bites.
People living in villages are at risk of snakebite because their housing conditions are poor and inadequate lighting makes it easy for the snakes to access their living spaces. Open-style habitation, open defecation and the sleeping on the floor often expose people to bites.

4/N
Snakebites are often associated with failure of vital organs and most rural hospitals lack the intensive care facilities required for the care of patients with multi-organ dysfunction. Antivenom is expensive (Rs 5000-10 000), and its inappropriate use in hospitals is common.
Snakebites are often associated with failure of vital organs and most rural hospitals lack the intensive care facilities required for the care of patients with multi-organ dysfunction. Antivenom is expensive (Rs 5000-10 000), and its inappropriate use in hospitals is common.
5/N
The “big 4” venomous snakes in India are common cobra (Naja naja), Russell's viper (Dabiola russelii), saw-scaled viper (Echis carinatus) & common krait (Bungarus caeruleus).
The snake venom is a cocktail of enzymes, polypeptides, glycoproteins; 90% snake venom is protein.
The “big 4” venomous snakes in India are common cobra (Naja naja), Russell's viper (Dabiola russelii), saw-scaled viper (Echis carinatus) & common krait (Bungarus caeruleus).
The snake venom is a cocktail of enzymes, polypeptides, glycoproteins; 90% snake venom is protein.
6/N
Snake venoms predominantly cause paralysis (Cobras and Kraits) or do not allow the blood to clot (Vipers).
Snake venoms predominantly cause paralysis (Cobras and Kraits) or do not allow the blood to clot (Vipers).
7/N
Patients come to hospitals with:
“Dry bite” (a venomous snake bites a person & the victim does not suffer from any signs or symptoms of envenomation), or bite by a non-venomous snake. 50% bites by Russell’s vipers, 30% by cobras, and 10% by saw-scaled vipers are dry bites.
Patients come to hospitals with:
“Dry bite” (a venomous snake bites a person & the victim does not suffer from any signs or symptoms of envenomation), or bite by a non-venomous snake. 50% bites by Russell’s vipers, 30% by cobras, and 10% by saw-scaled vipers are dry bites.

8/N
Local envenoming: Fang marks, local pain (burning, bursting, throbbing), swelling that extends up the limb, enlarged and tender lymph nodes draining the site of the bite, blistering, necrosis and local bleeding. Krait bites are typically painless.
Local envenoming: Fang marks, local pain (burning, bursting, throbbing), swelling that extends up the limb, enlarged and tender lymph nodes draining the site of the bite, blistering, necrosis and local bleeding. Krait bites are typically painless.
9/N
Systemic envenoming: Nausea, vomiting, abdominal pain, weakness, drowsiness.
Heart (Viperidae): dizziness, collapse, shock, abnormal heart rhythm, wet lungs.
Bleeding and clotting disorders (Viperidae):
●Bleeding from recent wounds
●Nose, gum, stomach bleeds
Systemic envenoming: Nausea, vomiting, abdominal pain, weakness, drowsiness.
Heart (Viperidae): dizziness, collapse, shock, abnormal heart rhythm, wet lungs.
Bleeding and clotting disorders (Viperidae):
●Bleeding from recent wounds
●Nose, gum, stomach bleeds
10/N
Neurological (Cobra and Krait): Droopy eyes usually within a few hours of the bite. Double vision, difficulty breathing, difficulty swallowing and respiratory muscle weakness follows. A few patients require mechanical ventilation, often for weeks
Neurological (Cobra and Krait): Droopy eyes usually within a few hours of the bite. Double vision, difficulty breathing, difficulty swallowing and respiratory muscle weakness follows. A few patients require mechanical ventilation, often for weeks
11/N
EARLY CLUES THAT A PATIENT HAS SEVERE ENVENOMING
●Rapid early extension of local swelling from the site of the bite
●Early tender enlargement of local lymph nodes
●Early systemic symptoms:
oLow BP
oVomiting
oHeavy eyelids, Droopy eyes,
oNose bleed, gum bleeding
EARLY CLUES THAT A PATIENT HAS SEVERE ENVENOMING
●Rapid early extension of local swelling from the site of the bite
●Early tender enlargement of local lymph nodes
●Early systemic symptoms:
oLow BP
oVomiting
oHeavy eyelids, Droopy eyes,
oNose bleed, gum bleeding
12/N
The 20-min whole blood clotting test, a cheap and simple test, is the most commonly used test to quickly diagnose venomous snakebite and administer antivenom.
The 20-min whole blood clotting test, a cheap and simple test, is the most commonly used test to quickly diagnose venomous snakebite and administer antivenom.
13/N
A few milliliters of blood is placed in a new, dry glass test tube. After 20 minutes, the tube is tipped to assess if a clot has formed. The test is negative if a clot is formed and positive if the blood is still liquid and unclotted.
A few milliliters of blood is placed in a new, dry glass test tube. After 20 minutes, the tube is tipped to assess if a clot has formed. The test is negative if a clot is formed and positive if the blood is still liquid and unclotted.

14/N
ANTIVENOM TREATMENT
Antivenoms are often unavailable or unaffordable for the poor, rural people most likely to be bitten. Most PHCs either do not stock enough antivenom or administer only a tenth of the full dose—a common practice.
ANTIVENOM TREATMENT
Antivenoms are often unavailable or unaffordable for the poor, rural people most likely to be bitten. Most PHCs either do not stock enough antivenom or administer only a tenth of the full dose—a common practice.
15/N
ANTIVENOM: DO’S AND DON’TS
●Use ASV only when its benefits exceed the adverse effects.
●Avoid inappropriate use of ASV.
●Do not use skin tests to predict ASV reactions.
●Ensure that epinephrine is available at the bedside before ASV is administered.
ANTIVENOM: DO’S AND DON’TS
●Use ASV only when its benefits exceed the adverse effects.
●Avoid inappropriate use of ASV.
●Do not use skin tests to predict ASV reactions.
●Ensure that epinephrine is available at the bedside before ASV is administered.
16/N
ANTIVENOM: DO’S AND DON’TS
Do not administer ASV locally and by intramuscular route.
●Observe patients for at least one hour after injecting ASV.
●Children should receive exactly the same dose of ASV as adults.
●Do not rely on ASV alone to treat Krait bite.
ANTIVENOM: DO’S AND DON’TS
Do not administer ASV locally and by intramuscular route.
●Observe patients for at least one hour after injecting ASV.
●Children should receive exactly the same dose of ASV as adults.
●Do not rely on ASV alone to treat Krait bite.
17/N
WHEN ANTIVENOM TREATMENT?
Antivenom should be administered as soon as possible as it is effective only against freely circulating venom.
Inject Antivenom whenever there are signs of systemic envenomation or presence of severe local swelling.
WHEN ANTIVENOM TREATMENT?
Antivenom should be administered as soon as possible as it is effective only against freely circulating venom.
Inject Antivenom whenever there are signs of systemic envenomation or presence of severe local swelling.
19/N
DOSAGE OF ANTIVENOM
The response to antivenom is highly dependent on the toxins of the biting snake and the severity of envenoming. In practice, initial dose is often chosen empirically.
Patients with Cobra, Viper or Krait envenoming requires 100-200 ml. (10-20 vials).
DOSAGE OF ANTIVENOM
The response to antivenom is highly dependent on the toxins of the biting snake and the severity of envenoming. In practice, initial dose is often chosen empirically.
Patients with Cobra, Viper or Krait envenoming requires 100-200 ml. (10-20 vials).
20/N
How do we know that antivenom has worked?
If after antivenom infusion (1) blood pressure becomes normal, (2) bleeding stops, (3) blood begins to clot again and (4) nerves begin to function normally.
Antivenom may not reverse respiratory muscle paralysis caused by Kraits.
How do we know that antivenom has worked?
If after antivenom infusion (1) blood pressure becomes normal, (2) bleeding stops, (3) blood begins to clot again and (4) nerves begin to function normally.
Antivenom may not reverse respiratory muscle paralysis caused by Kraits.
21/N
When do we repeat the antivenom?
1.Blood has not clotted — on a bedside 20-minute blood clotting test—after 6 hours of the first dose of antivenom.
2. Heart and nervous system dysfunction after 1 hour of antivenom.
When do we repeat the antivenom?
1.Blood has not clotted — on a bedside 20-minute blood clotting test—after 6 hours of the first dose of antivenom.
2. Heart and nervous system dysfunction after 1 hour of antivenom.
22/N
How do we prevent antivenom reactions?
0.25 mg adrenaline given subcutaneously before ASV administration is safe and reduces the risk of acute severe reactions to ASV.
Anti-histamines and steroids are ineffective for this purpose.
How do we prevent antivenom reactions?
0.25 mg adrenaline given subcutaneously before ASV administration is safe and reduces the risk of acute severe reactions to ASV.
Anti-histamines and steroids are ineffective for this purpose.
23/N
Cobra differs from Krait!
Cobra: Improvement in paralytic signs may become clinically evident within 30–60 minutes of an initial dose of 10 vials of antivenom.
Krait: No evidence to justify the prolonged administration of antivenom in paralyzed and ventilated patients.
Cobra differs from Krait!
Cobra: Improvement in paralytic signs may become clinically evident within 30–60 minutes of an initial dose of 10 vials of antivenom.
Krait: No evidence to justify the prolonged administration of antivenom in paralyzed and ventilated patients.
24/N
ACUTE KIDNEY INJURY
A fifth of patients develop acute kidney injury following a venomous snakebite. Cautious intravenous fluids, diuretics, and haemodialysis or peritoneal dialysis may be required in acute kidney injury.
ACUTE KIDNEY INJURY
A fifth of patients develop acute kidney injury following a venomous snakebite. Cautious intravenous fluids, diuretics, and haemodialysis or peritoneal dialysis may be required in acute kidney injury.
25/N
Sleeping under a mosquito net and on a bed above ground level, keeping domestic areas free of rubbish, rubble, and firewood and controlling rodent populations are vital to prevent deaths from snakebites.
Sleeping under a mosquito net and on a bed above ground level, keeping domestic areas free of rubbish, rubble, and firewood and controlling rodent populations are vital to prevent deaths from snakebites.

Two wishes.
(1) Can Indian scientists develop point of care molecular tests to help care providers identify the species responsible for snakebites? Accurately, quickly and cheaply?
@doctorsoumya @ICMRDELHI
(1) Can Indian scientists develop point of care molecular tests to help care providers identify the species responsible for snakebites? Accurately, quickly and cheaply?
@doctorsoumya @ICMRDELHI
Can Government develop monovalent antisnake venom for the big four (Russell’s and Saw-scaled Viper, Cobra and Krait) to improve the efficacy of antivenom? And ensure its adequate availability?
Thousands of lives can be saved if we achieve these two goals.
#SaveLives #snakebite
Thousands of lives can be saved if we achieve these two goals.
#SaveLives #snakebite
• • •
Missing some Tweet in this thread? You can try to
force a refresh