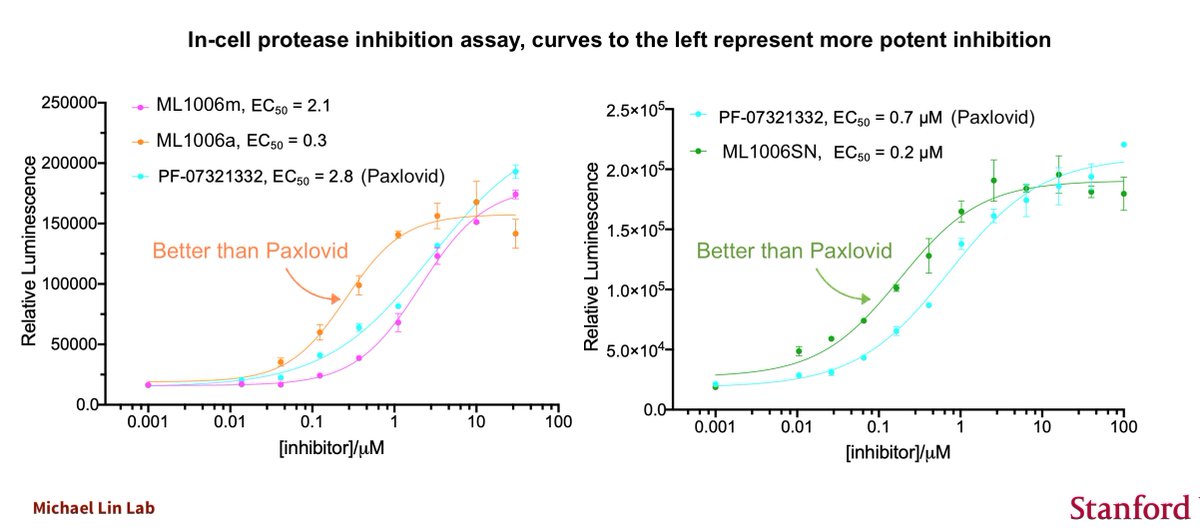
Will continue with Pfizer vs Moderna at another time... I have papers to write and need to keep the lab running, train students, etc, and don't get paid for the COVID-19 stuff
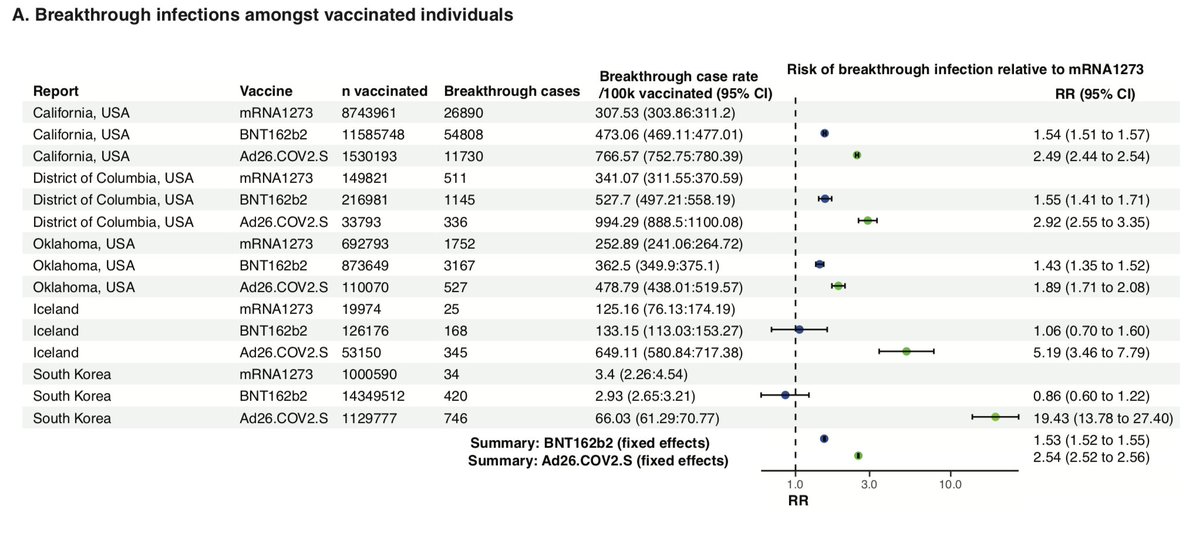
OK and DC numbers were all-time, so both pre-∆ and ∆. They don't account for RNA vaccines being given when J&J was not yet approved or on hold, but that effect is minor now that the average exposure risk experienced by recipients become more similar over time.
In San Diego, the data are recent, so mostly Delta. The 23 unvaccinated vs 2.3 J&J vaccinated cases per 1000 people would suggest 90% efficacy. This of course would be really great. But could be vaccinated are also more careful, so it's hard to know without a case-control study
By combining the data in the top post, I made the assumption any protection drop would be similar for the Delta subset as the non-Delta subset. I think it's reasonable as disease protection is mostly antibody mediated, and Delta evades antibodies from all vax to the same degree
So another way to estimate Delta protection is to scale from the RNA vaccine efficacy in case-control study. Pfizer showed 80-88% effectiveness vs Delta in such studies from England and Scotland (NEJM and Lancet papers)
Scaling that 12-20% breakthrough vs unvaccinated for Pfizer on Delta by 150% predicts 18-30% breakthrough vs unvaccinated for J&J on Delta, giving 70%-82% protection. That's actually pretty good.
It's a bit hard to reconcile with the 71% protection against hospitalization in the SA Sisonke trial, but that was unblinded so the vaccinated may have been more willing to take risks, and that was in HCWs so they may have a low threshold for hospitalization. Hard to say.
The right way to evaluate a claim in biorxiv/medrxiv is to read the paper. If you find something wrong with it, then you can dismiss it. Dismissing papers only because they haven't completed peer review often backfires, as posted findings tend to be true. dailymail.co.uk/news/article-9…
I haven't had time to read this paper but it does fit with other observations as well as the fact that Moderna's vaccine uses 3.3x the RNA as Pfizer's, so I see no need to dismiss it yet
Okay I guess I talked myself into reading this paper, since I just said one should read the paper before dismissing it. But I wouldn't want that comment to be misinterpreted to mean I would accept the findings without reading it either.
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
Verdict is: this is a large, well executed study in case-control format where they find matched triplets of Pfizer, Moderna, or unvaccinated, with similar age, sex, ethnicity, and state, and between Pfizer and Moderna vax dates within 2 week. 

There were 45k triplets in USA, 25k in MN. In 22k MN triplets, vax recipients were fully vaxxed, so these were analyzed. KM curves for protection show 56% fewer breakfthroughs in Moderna (orange) vs Pfizer (blue). CI do not cross 0%, and p value is significant at 0.0034. 

This analysis was extended to all fully vaxxed triplets in their US database, and the effect held up, with Moderna showing relative risk reduction of 0.50 vs Pfizer (95% CI 0.39 to 0.64). 

The results are similar between Jan and July and in July only, suggesting the increased protection applies for Delta which is dominant in July
The paper actually rather unsells its main point. The 0.50 risk reduction of Moderna vs Pfizer is mentioned in the abstract, but only after a mind-numbing recitation of each vaccine’s efficacy compared to nonvaccinated at different times. Hurts your eyes to read: 

Everything before you get to (IRR - 0.50) isn’t the main point of the paper which is the comparison between the two vax, title being “Comparison of two highly-effective mRNA vaccines”. And the 0.50 risk reduction doesn’t come with a graph.
On the other hand there is the KM plot for the 0.56 better protection by Moderna vs Pfizer in MN, but there is no mention of that in the abstract. It's like they almost went out of their way to make the interesting point — Moderna looks more protective than Pfizer — hard to find.
Anyway the finding that Moderna seems to have about half the breakthroughs of Pfizer looks robust, across states and applicable to Delta. Verdict on this study: not dismissable.
In addition, this finding is consistent with what we see in other regional statistics: Moderna recipients have a reduced rate of breakthroughs compared to Pfizer recipients. Collecting images from upthread: 





So we can, based on actual findings, assign relative efficacies of the vaccines as Moderna > Pfizer > J&J. The first two looked very similar when both produced more than enough antibody to neutralize the original strain they were designed to.
But with declining antibody titers over time and Delta's ability to partially escape Ab binding (requiring ~5x higher titers) plus its more symptomatic disease, the mean differences in antibody titer elicited by the Moderna and Pfizer vaccines become functionally relevant.
New UK study: Pfizer VE drops from 85% to 75% (12% relatively) in 3mo. If linear, then expect 65% at 6mo and 55% at 9mo.
There were concerns about Israel's calculation of 39% VE after 6mo as the methods weren't revealed, but today's study looks robust.
ndm.ox.ac.uk/covid-19/covid…
There were concerns about Israel's calculation of 39% VE after 6mo as the methods weren't revealed, but today's study looks robust.
ndm.ox.ac.uk/covid-19/covid…
They looked at positive vs negative tests in subpopulations defined by age or previous infection or preexisting conditions.
This controls for the effect of rising background immunity in nonvaxxed, and waning is also seen in both younger and older groups
@ImmunoFever
This controls for the effect of rising background immunity in nonvaxxed, and waning is also seen in both younger and older groups
@ImmunoFever

The projected ~55% VE after 9 months may be why US officials just suggested 9mo as the boosting time.
Also: Delta grows to similar titres in vaxxed as nonvaxxed. Below, lower Ct = more virus. Left set, pre-Delta. Right set, Delta. 1st/4th bar in each set = unvaxxed/vaxxed.
Also: Delta grows to similar titres in vaxxed as nonvaxxed. Below, lower Ct = more virus. Left set, pre-Delta. Right set, Delta. 1st/4th bar in each set = unvaxxed/vaxxed.

So if you're symptomatic with Delta, you're contagious vaxxed or not.
This unfortunately means previous hopes that breakthrough cases might not be that contagious with Delta, already refuted by contact tracing, is now also refuted by mechanism
This unfortunately means previous hopes that breakthrough cases might not be that contagious with Delta, already refuted by contact tracing, is now also refuted by mechanism
https://twitter.com/profshanecrotty/status/1411059639249084416
Another note is AZ efficacy is clearly lower. So VE estimates compared between trials (which PH officials don't like to do) have held up in real life too. Note 1-dose J&J efficacy in trials was similar to 2-dose AZ, except for Beta where AZ was really bad for some reason.
A lot has happened in Oklahoma in 3 weeks. We've seen a many-fold increase in the number of breakthrough infections. This was due to the Delta wave, of course.
It means we now have enough numbers to derive breakthrough hosp and death rates that are statistically meaningful.
It means we now have enough numbers to derive breakthrough hosp and death rates that are statistically meaningful.
Also the fact that most breakthroughs occurred in the last 3 weeks means we no longer have to worry much about the effect of J&J being unavailable for 2 months in early 2021. By now most people have been vaccinated for about the same amount of time.
And the results are that J&J hospitalization and death rates are trending about 2x higher than Moderna, with Pfizer in between (my calculations in red). This is similar to the relative risk of being a case for J&J vs Moderna and Pfizer. 

As the SA Sisonke trial estimated J&J's protection against Delta hospitalization at 71%, which sees quite different from the UK estimates of >90% protection by Pfizer, we kind of expect that J&J hospitalization protection might be weaker than Pfizer or Moderna in the same popn.
These are the first results I know where there are enough hospitalization events to analyze. Fisher's exact says the diff between J&J and Moderna, or J&J and Pfizer, is statistically significant. Alpha is 0.025 to adjust for 2 tests to meet the (arbitrary) 95% significance level. 

Updated breakthrough case info from DC
Interesting: vaxxed vs unvaxxed case rates now reported at the top. Maybe was there earlier and I missed it. It's very informative
14.2 vaxxed vs 47.9 unvaxxed cases per 100k means relative risk of 30%, meaning VE=70% across all vax
Interesting: vaxxed vs unvaxxed case rates now reported at the top. Maybe was there earlier and I missed it. It's very informative
14.2 vaxxed vs 47.9 unvaxxed cases per 100k means relative risk of 30%, meaning VE=70% across all vax

But that this overall VE is an underestimate, as some % of unvaxxed are immune from prior infection. I estimate for DC this is ~1 in 5. So that means the 48 unvaxxed cases happened in the 80% unvaxxed plus 5% of the 20% vaxxed due to breakthrough (estimated recursively)
So the rate per 100k unvaxxed can be estimated as 48/0.85 = 56. So then adjusted VE is 1-14/56 = 75%.
Okay then what's really interesting is the relative breakthrough % at the bottom allows an estimate of VE for J&J, for the first time
Okay then what's really interesting is the relative breakthrough % at the bottom allows an estimate of VE for J&J, for the first time
The breakthrough rates relative to unvaxxed are then 50%, 26.5%, and 17% for J&J, Pfizer, and Moderna respectively.
This makes estimated VE of 50%, 74%, and 83% for J&J, Pfizer, and Moderna in Washington DC
This makes estimated VE of 50%, 74%, and 83% for J&J, Pfizer, and Moderna in Washington DC

Graphic with the Moderna/Pfizer numbers unflipped:
Vaccine effectiveness =
50% J&J
74% Pfizer
83% Moderna
In the Washington DC population with vast majority f infections since July 1, representing essentially all Delta
Vaccine effectiveness =
50% J&J
74% Pfizer
83% Moderna
In the Washington DC population with vast majority f infections since July 1, representing essentially all Delta

Moderna has 2x higher Ab titers than Pfizer. Not surprising, as we've established that it's more efficacious than Pfizer in preventing cases in the real world.
https://twitter.com/EricTopol/status/1432436808042749952
Also not surprising as a 2x difference between Moderna and Pfizer was estimated in the below Nature Medicine paper. Today's result validates the NM study.
Note the NM study also estimates 8x lower antibody levels for J&J vs Moderna (4x vs Pfizer)
nature.com/articles/s4159…
Note the NM study also estimates 8x lower antibody levels for J&J vs Moderna (4x vs Pfizer)
nature.com/articles/s4159…
Adding updated info from OK. I decided to calculate what % of cases are hospitalized for each vax. Results are similar for all 3 vax. Thus no evidence that a better T cell response in J&J is leading to less progression from infection to hospitalization from Delta. 

J&J effectiveness against Delta disease revealed to be "about half" in South Africa trial.
This fits my 50% estimate from DC data above
This fits my 50% estimate from DC data above
https://twitter.com/michaelzlin/status/1435645689040551936
Colorado figures match DC and South Africa.
Relative protection against disease roughly 80/70/50% Moderna/Pfizer/J&J
Click "state level" then "vaccine specific" at covid19.colorado.gov/vaccine-breakt…
Thanks
@CescaRose80
Relative protection against disease roughly 80/70/50% Moderna/Pfizer/J&J
Click "state level" then "vaccine specific" at covid19.colorado.gov/vaccine-breakt…
Thanks
@CescaRose80

New data from a CDC study of 9 states (including CO but not OK) shows what we have already concluded: J&J protection vs hospitalization is lower than the other two vaccines
https://twitter.com/michaelzlin/status/1436393327604105218
Making progress getting these numbers into the public discussion
https://twitter.com/EricTopol/status/1437409399232811009?s=19
Today's news: Due to severe cases being disproportionately #JnJers, France appeals to all J&J recipients to boost with RNA if ≥4 weeks post-vax.
Source:
Translation in pics. Note Google wrote appelée="required", better as "appealed to"
h/t @maggiemae362

Source:
https://twitter.com/nicolasberrod/status/1437406466462339073
Translation in pics. Note Google wrote appelée="required", better as "appealed to"
h/t @maggiemae362


@maggiemae362 Just saw LiveScience ran an article reporting the figures in the graphics in post #1 in this thread. The article appeared on August 11, the day after the post.
Great to see the info getting cited. Wish more journalists reported primary data. @NicolettaML
livescience.com/breakthrough-i…
Great to see the info getting cited. Wish more journalists reported primary data. @NicolettaML
livescience.com/breakthrough-i…
New medrxiv preprint: First to show stat sig differences etween all 3 FDA-approved vaccines in VE vs cases. It confirms what we already deduced above: Moderna > Pfizer > J&J
Also Moderna significantly > J&J for hospitalizations, with Pfizer in between
medrxiv.org/content/10.110…
Also Moderna significantly > J&J for hospitalizations, with Pfizer in between
medrxiv.org/content/10.110…

Review of all vaccines. Pre-Delta, but useful to recall baseline differences. As I've noted before, J&J VE for disease is similar to Sinovac/Sinopharm inactivated vaccines.
https://twitter.com/alison_l_hill/status/1443610104130015232
Interesting nugget in the FDA VRBPAC packet: followup of ENSEMBLE1 through July shows J&J VE for cases dropping to 56%. That would be a mix of Delta and non-Delta. Thus VE is approaching the 50% vs Delta cases I had estimated from breakthroughs above
fda.gov/media/153037/d…
fda.gov/media/153037/d…

This also suggests J&Js report on "real world" VE based on medical records was wrong. They claimed 79% VE vs infections through July, but they added 10% to correct for supposed underreporting of vaccination status. The ENSEMBLE RCT doesn't have such issues
https://twitter.com/michaelzlin/status/1440424671518728200
This medical records-based study also could be confounded by different rates of treatment-seeking behavior among different demographics who in turn can be associated with less or more vaccination or exposure. When the study normalized for this they got VE 56%, close to ENSEMBLE.
No press release from J&J about this long-term followup of a 40000-person RCT showing waning efficacy in real-world case protection, whereas they had put out two press releases on antibody measurements in <20 people and on the non-controlled medical records study. Hmm.
This paper was just updated last week with a metaanalysis of publicly available data on breakthrough cases/deaths/hospitalizations for all 3 vaccines. The Iceland and Korea numbers are quite terrible for J&J (near zero case protection for Korea) 
J&J also had several-fold higher death rates than the other two vaccines.
Interestingly these authors found 3 datasets in the US: CA, OK, and WA. We found OK and WA above. If anyone has the CA link, let me know.
So we had found 2 of 3 available datasets. CA could be recent
Interestingly these authors found 3 datasets in the US: CA, OK, and WA. We found OK and WA above. If anyone has the CA link, let me know.
So we had found 2 of 3 available datasets. CA could be recent

The lower efficacy of J&J even in homogenous countries like Iceland suggests that VE differences are not simply from J&J being given to people in riskier jobs or worse living conditions.
And if it were, those people definitely deserve better protection than what J&J is providing
And if it were, those people definitely deserve better protection than what J&J is providing
A recent VA study estimated J&J vaccine protection at only 3% 6mo after vaccination adjusting for confounds. However, not sure how that was calculated as it seems lower than level seen in any of the age groups. Worst group was <50 with 25% protection
medrxiv.org/content/10.110…

medrxiv.org/content/10.110…
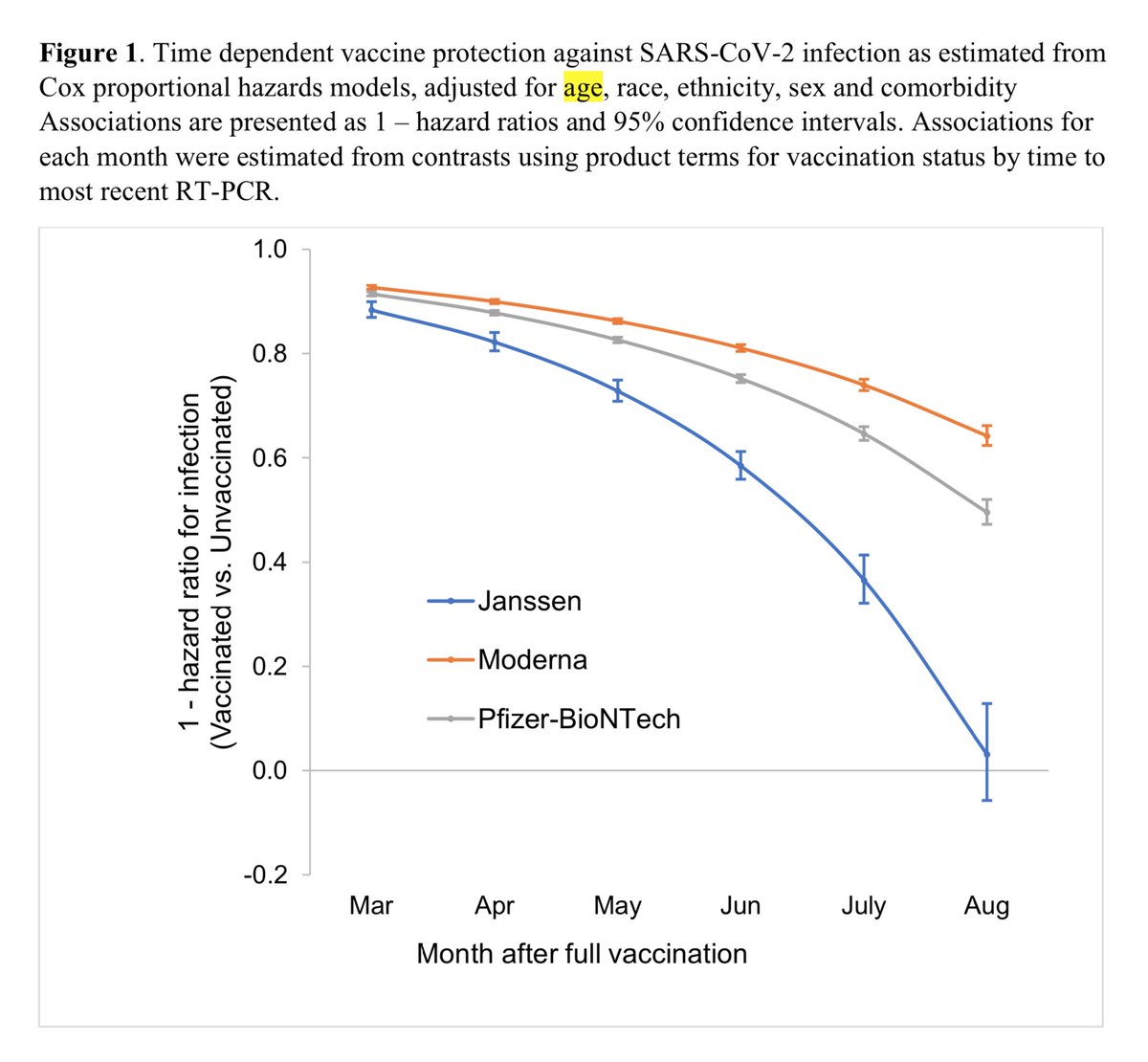

But regardless things seem to fall off a cliff pretty quickly after 5mo; this sigmoidal effect was already seen for the RNA vaccines as discussed in this thread
https://twitter.com/michaelzlin/status/1445967149369991171?ref_src=twsrc%5Etfw
Same rank order for breakthroughs in Oregon too: J&J > Pfizer > Moderna
By now, this should be beating a dead horse, but it could be a zombie horse, so worth beating.
h/t @MicheleB_PhD
By now, this should be beating a dead horse, but it could be a zombie horse, so worth beating.
h/t @MicheleB_PhD
https://twitter.com/EricTopol/status/1453506074871431169
Finally the @nytimes publishes the finding of breakthrough rates being J&J > Pfizer > Moderna
I wouldn't call the 2x higher cases for J&J vs Moderna, or the 4x (!) higher deaths, as "slightly higher", but happy to see *actual* graphs. About time.
nytimes.com/interactive/20…
I wouldn't call the 2x higher cases for J&J vs Moderna, or the 4x (!) higher deaths, as "slightly higher", but happy to see *actual* graphs. About time.
nytimes.com/interactive/20…

@nytimes BTW this definitively shows the "vaccinated don't need to mask indoors" guidance in May 2021 was a mistake. But of course any thinking person knew that then, since breakthroughs even happened on the original strain in the Phase 3 trial: 30% relative to the unvaxxed rate for J&J.
• • •
Missing some Tweet in this thread? You can try to
force a refresh