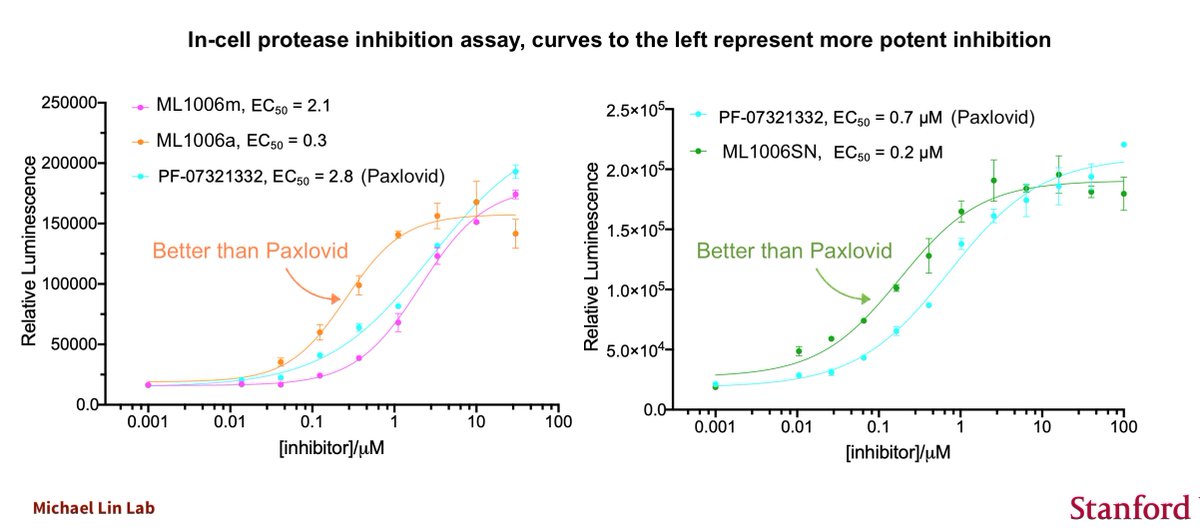
ICYMI, on 12/23, the last news day before a long holiday break, FDA approved the viral mutagen molnupiravir as an at-home COVID19 drug. It sounds worrisome because it is. I wrote in the @washingtonpost that immunoevasive variants could arise from its use.
washingtonpost.com/outlook/2021/1…
washingtonpost.com/outlook/2021/1…
If molnupiravir gives rise to enhanced mutants of SARSCoV2, it will prolong the pandemic and cause countless deaths and needless suffering. Yet it's only 30% effective in preventing hospitalization, similar to generic antidepressant and far worse than the 89% of other antivirals.
FDA knew of the concerns about mutant viruses escaping from patients taking MOV. This was discussed at the AMDAC FDA advisors meeting 1 month ago and contributed to the 10 no votes. It was revealed Merck didn't know what mutations occur in patients and when viruses are cleared.
Some reporters noted the unresolved issue of molnupiravir driving viral evolution. Most physician colleagues I spoke opposed molnupiravir approval. Reporters I spoke to were aware of the debate but felt they had nothing to report until FDA made a decision.
cnbc.com/2021/11/30/fda…
cnbc.com/2021/11/30/fda…

Well FDA has now made a decision, and distressingly, it was the wrong one, to grant EUA to molnupiravir. There is now news to cover. The most shocking thing about the EUA is that FDA explicitly recognizes that antibody-evading mutants could arise, and is allowing it to happen.
You can see the FDA awareness in 3 places in the EUA and Fact Sheet. First, FDA notes MOV has been shown to create mutations in antibody-binding sites on the SARSCoV2 spike protein. This causes immune evasion. But FDA writes, wrongly, "the public health significance is unknown"! 

FDA also says everyone should take all pills for 5 days and be isolated in the meantime to avoid transmission of mutant virus. But the drug can only be used outside of hospitals, and nobody will enforce at home. Nursing facilities could do it, but FDA didn't restrict to that. 

Finally, FDA is asking Merck to supply data on virus levels at the end of treatment (by PCR and by ability to infect cells), and sequences of mutations found, from trial participants. And they request similar info from immunocompromised who take longer to clear virus. 

So FDA knew this info, needed to assess risk, are missing. It should have been supplied *before* EUA. Yet FDA went ahead and approved without it. And it's been months since the trials, and not sure how well virus survived in those samples. Why did Merck not analyze them earlier?
The endpoint viral titers isn't the most important thing anyway; we need to know viral titers on days 1-2 of treatment, when virus is picking up mutations but viral titers don't fall (they are not significantly different from placebo through day 3) science.org/doi/10.1126/sc… 

The FDA decision was clearly made in haste, with important outstanding issues not settled. The best we can do now is raise awareness of the risks, to prescribe MOV only when isolation is assured (living alone or in SNFs), and push for EUA reversal if the risks are not addressed.
Now one of the things you often hear is "I can't understand the science and don't know whether to believe Merck or some Stanford professor". First of all, most of my colleagues, and all of them who have actually looked at the data or lack thereof, think EUA is a bad idea.
Second, this is not just a scientific issue. In fact, as a drug to treat a pandemic with a risk of making it worse, it is primarily *not* a scientific issue. We don't have lab scientists (PhDs, generally) making decisions about whether and when to dispense drugs. That's a MD role
MDs have an oath to "first, do no harm". If molnupiravir has some risk of harm, and everyone from its most ardent supporters to the FDA acknolwedges that, then it's no longer just an esoteric scientific debate. It is at the very least a medical debate. So MDs should get involved.
Second, accelerated evolution of new variants created by a drug would clearly necessitate public health, economic, and political responses. Thus molnupiravir is a topic government officials outside of FDA and anyone involved in government policy and advising should discuss too.
Thus it's appropriate to point out here that my concerns are shared by prominent government advisors or former officials (current officials of course can't speak against FDA). HIV pioneer Bill Haseltine was the first to warn about the risks of molnupiravir
forbes.com/sites/williamh…
forbes.com/sites/williamh…
FDA advisor Dr. James Hildreth @JamesEKHildreth is dismayed by the EUA as well.
He was 1 of 10 AMDAC members voting no on the benefit/risk question, and 1 of 6 raising the risk of escaped mutated virus, even though they weren't asked to comment about it.
He was 1 of 10 AMDAC members voting no on the benefit/risk question, and 1 of 6 raising the risk of escaped mutated virus, even though they weren't asked to comment about it.
https://twitter.com/JamesEKHildreth/status/1474083223542149124
Former BARDA director (and CDC immunologist and virologist) Rick Bright @RickABright also supports my concerns about patients not completing therapy and thus harboring viable mutant viruses, and the importance of the missing mutation data from Merck 



Sociologist Zeynep Tufecki @zeynep whom many of us know well for her uncanny ability to discern a rigorous argument from groupthink, has also deemed the concerns worthy of debate and discussion
https://twitter.com/zeynep/status/1467888468831481858
Also any particular person's lack of comment on molnupiravir should not be taken as support for FDA's decision at this time. Since EUA on 12/23, many/most people have been traveling, visiting family, shopping, recuperating from a difficult year, or reading/writing about omicron.
Merck's first human data release (data, not top-line news) occurred in the FDA docs on 11/29, which were followed by the AMDAC meeting the next day. Not only was that right after Thanksgiving, it was also when Omicron first arose and everyone was busy dealing with that.
We then spent all of December trying to figure out how bad Omicron would be, on top of typical end-of-year duties for work and family. There was simply no time or opportunity for most people to read and think about molnupiravir in detail, let alone write something cogent about it
But note 2 out of 2 attempts to argue against my viewpoints by appeals to another authority ended up with them supporting my concerns 

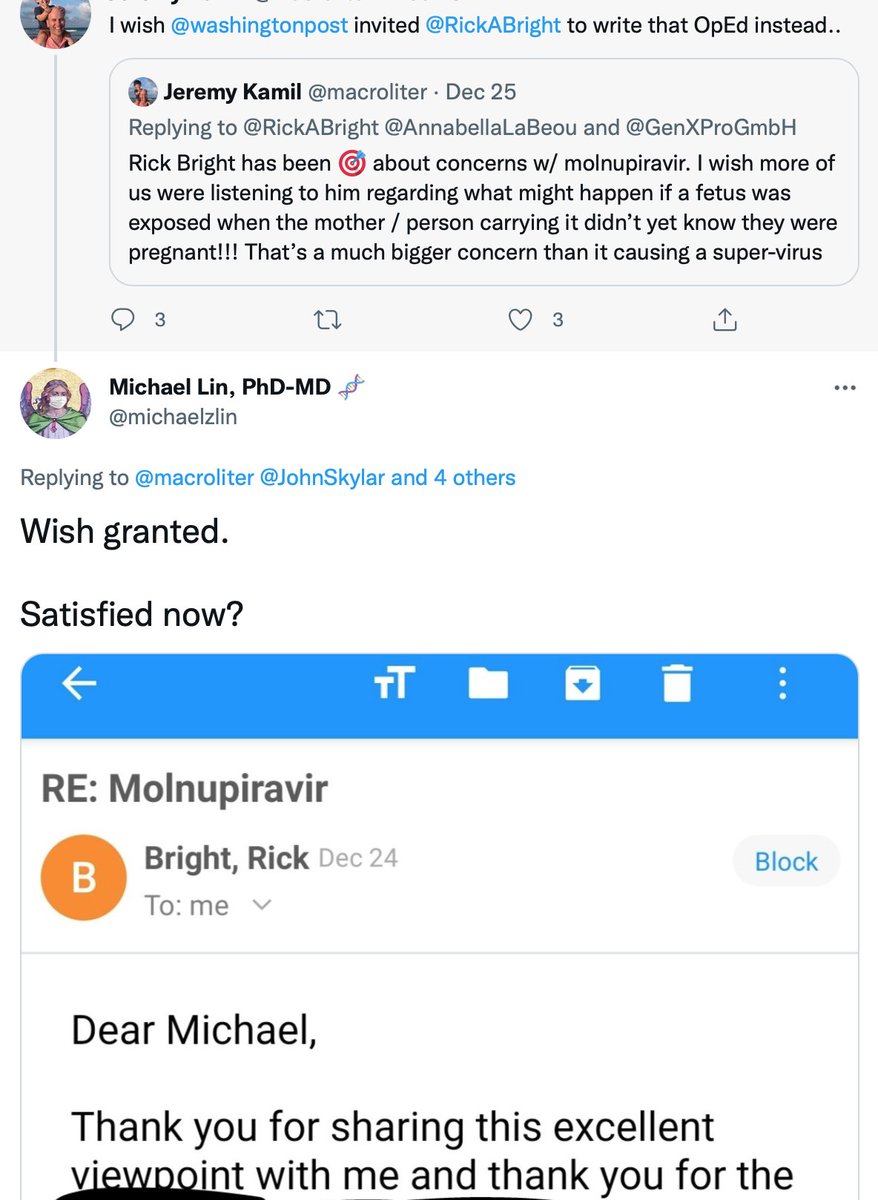

Point is, if you ask doctors or scientists who were *not* involved in developing molnupiravir, you're likely to find (1) some aren't aware of the data as it was not publicized in the press or available before this week, and (2) those aware are mostly opposed to EUA at this time.
Thus we just witnessed a rushed stealth EUA: Merck released what little data they had days after Omicron, FDA made a decision within a month while the press was busy dealing with Omicron, and FDA released the EUA the day before the most important holiday week of the year.
Again this doesn't mean that everybody has been unaware or that I am a pioneer viewpoint; besides @WmHaseltine sounding the alarm we have @sarperotto and @chasewnelson detailing concerns as far back as 11/8 with support from @CT_Bergstrom.
https://twitter.com/sarperotto/status/1457781777154514949
I've learned a lot from these and opposing viewpoints, and reading the available data carefully, and had started a thread explaining everything when the EUA unexpectedly hit. That's the only reason my article could be the first out after EUA, but it won't be the last.
That thread (135 posts, a 30-min read) is linked below in threadreader format. In it you'll find more details about how molnupiravir approval appears to be a historical accident, and why arguments supporting it all fail to consider some important factor.
threadreaderapp.com/thread/1473818…
threadreaderapp.com/thread/1473818…
But I know that thread is very long, so I'll link to a few useful parts of it. If you hear arguments presented about why my concerns are wrong, please start reading the thread here:
https://twitter.com/michaelzlin/status/1474477641751162881
Essentially I've analyzed each of them and found all lacking some essential understanding, e.g. on drug biodistribution, the lack of viral suppression early in treatment, the unprecedented use of a mutagen on a highly contagious disease, that rare events will amplify like Omicron
Finally I'll mention our funding sources. We get funding for 1 scientist from private donors to work on antiviral drugs. Most of our funding is for pre-COVID topics. I take no money from drug companies for COVID. More details here:
https://twitter.com/michaelzlin/status/1474463707656687617
And once again, everyone, ribavirin is not precedence for MOV. It's a myth that it works by viral mutagenesis; data show it most likely works by boosting innate immune responses. Also it's never been used in something as explosively contagious as COVID19.
https://twitter.com/michaelzlin/status/1475239040886591488
• • •
Missing some Tweet in this thread? You can try to
force a refresh