
1/ Cells make, regulate and break down proteins constantly. They have a system to control the regulation of the proteins they produce. This is to remove unwanted proteins when no longer used.
2/ It also maintains healthy proteins as they degrade slowly over time. The process of ubiquitination is the tagging of these proteins by the cell for destruction. There are 3 enzymes that work in the process of ubiquitination. 

3/ They are the enzymes E1, E2 and E3. There are only 2 types of E1 enzymes, 30 types of E2 enzymes and estimated to be 600 possible E3 enzymes. Lets take a look at how a protein get tagged for destruction. 
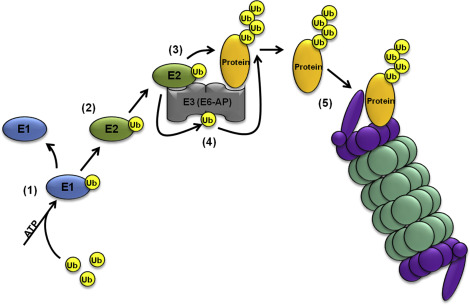
4/ The E1 ligase take the actual ubiquitin molecule and puts a phosphate on it. This is called phosphorylation. Once its got that phosphate group added, it passes the ubiquitin to the E2 enzyme.
5/ The E2 ligase takes the phosphorylated ubiquitin and binds to the E3 ligase with it. This creates a complex of the E2 and E3 ligases. The E3 ligase will bind to the protein it recognizes and add that ubiquitin to the protein.
6/ The addition of the ubiquitin to the protein is done on a lysine amino acid. It can be done to several lysine amino acids on that same protein by multiple E3 ligases.
7/ After the E3 adds the ubiquitin to the protein, it releases the E2 ligase and binds another which contains another ubiquitin.
8/ It keeps adding these ubiquitin molecules to the lysine in a long chain called a poly ubiquitin chain. It can create a chain of more than 10 ubiquitin molecules long.
9/ This long tail of ubiquitin on the protein flags it for destruction by the organelle called the Proteasome.
• • •
Missing some Tweet in this thread? You can try to
force a refresh





