Agenda posted for FDA advisory committee meeting Friday on #covid19 boosters. Departing regulator Dr Marion Gruber kicks off the morning with an introduction of the topic (after this week co-authoring letter saying boosters not needed): fda.gov/media/152159/d… 

Also interesting, on FDA’s agenda for Friday booster shot advisory mtg: presenters from UK & Israel on real-world evidence 

In its briefing docs for Friday’s FDA booster mtg, Pfizer lays out the case for boosting at 6-8 months, citing data from Israel & US. Notes the erosion is likely due more to waning effectiveness than Delta escape: fda.gov/media/152161/d… 

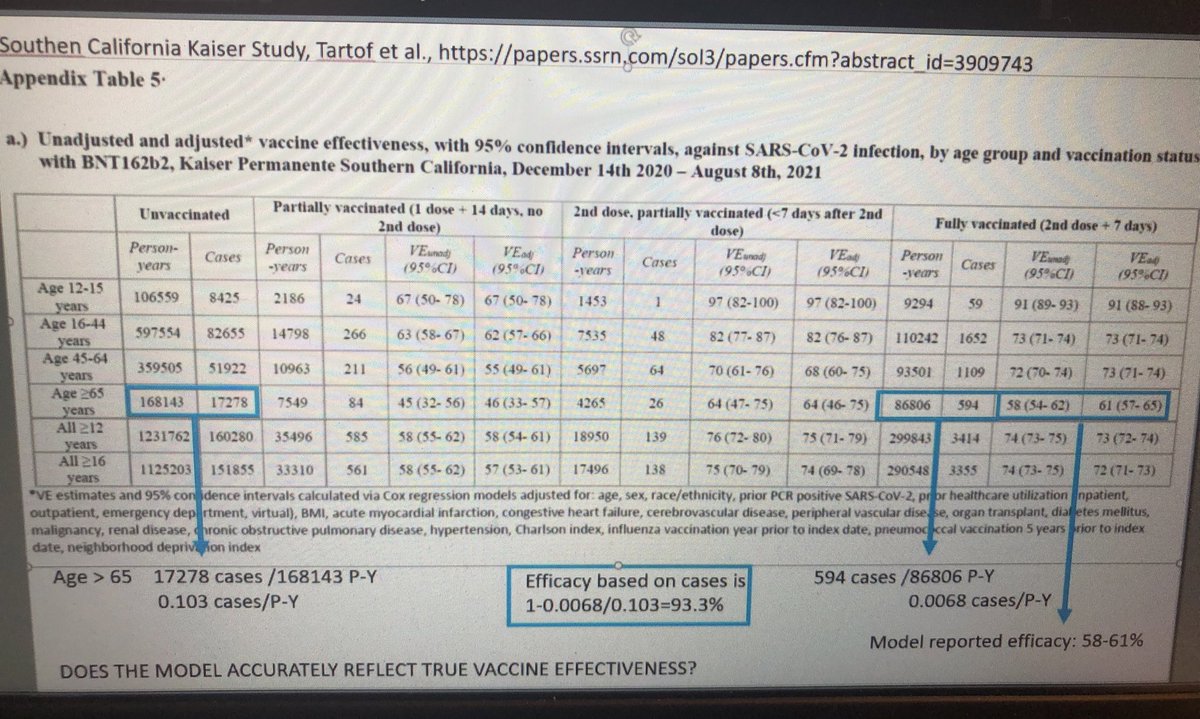
Alarming numbers here from Israel in Pfizer briefing docs ahead of Friday FDA mtg on boosters. Key is last point, on protection against severe disease: for 65+, dropped to <60% in late June/Aug for those vaccinated in Jan/Feb: 

• • •
Missing some Tweet in this thread? You can try to
force a refresh