FDA booster meeting Q&A begins w Dr Marion Gruber turning it over to Dr Phil Krause, who notes much of the data being discussed today is "not peer-reviewed and has not been reviewed by FDA."
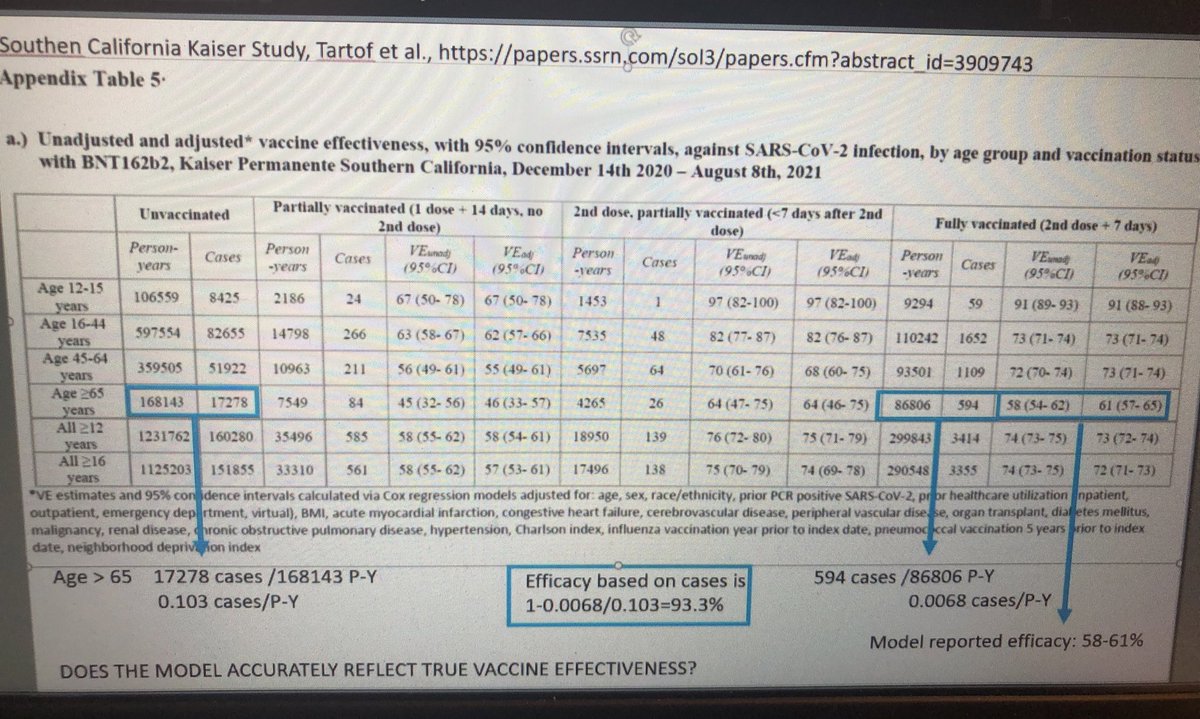
Asks Pfizer whether Kaiser model accurately reflects true vaccine effectiveness
Asks Pfizer whether Kaiser model accurately reflects true vaccine effectiveness
And Pfizer is having crazy audio issues responding to Dr Krause
I am feeling so awkward
I am feeling so awkward
VRBPAC chair moves on quickly from Krause questions to Pfizer on Kaiser model, noting there are often statistical questions like this and apologizes to voting members for cutting into their Q&A time
Pfizer rapidly escalating who is answering questions from VRBPAC, now up to Dr Kathrin Jansen, head of vaccine R&D (whose audio also has been the best so far from the company)
VRBPAC's Dr Cody Meissner asks why we wouldn't take cues from flu in updating vaccines seasonally and update #covid19 boosters to circulating variants
Pfizer continues to have audio issues but says "we've not yet seen a variant" that escapes the vaccine
Pfizer continues to have audio issues but says "we've not yet seen a variant" that escapes the vaccine
Pfizer’s Dr Bill Gruber cites this slide in responding to Dr Meissner’s Q about whether vaccine should be updated to target delta specifically (Pfizer perspective, based on this: no need) 

VRBPAC's Dr Mark Sawyer notes ~300-person database from Pfizer "strikes me as a little bit small" for assessing safety of a booster dose, asks FDA about safety database sizes for comparable vaccine boosters for other diseases
FDA's Dr Doran Fink notes 300 is on lower end of experience with boosters for other diseases, which range from about 300 to 1,000 with varying levels of post-marketing surveillance, but notes large database of clinical trials supporting first 2 doses for #covid19
VRBPAC's Dr Ofer Levy asks whether Israel has experience with myocarditis for ages 16-18; Dr Sharon Alroy-Preis says appears lower rate than after 2nd dose, notes Israel is doing active surveillance for myocarditis and has just 1 case (30 yr old), but not yet 30 days out
FDA's Dr Peter Marks: "It's no secret here that there is still debate over the need for an additional #covid19 vaccine at this stage of the pandemic, but the emerging evidence from our Israeli colleagues is very helpful."
VRBPAC chair asks whether committee allowed to use any data from outside Pfizer trial when making its vote on booster. Dr Gruber answers first but Dr Marks steps in...
(Recall, Drs Gruber & Krause are retiring this fall, in part reportedly due to frustrations over this process)
(Recall, Drs Gruber & Krause are retiring this fall, in part reportedly due to frustrations over this process)
Dr Marion Gruber says please look at Pfizer trial (as voting question specifies) but can use additional information to inform it
Dr Marks then adds - use the totality of the evidence before you. "The person that ignores data is the one that's surprised."
Dr Marks then adds - use the totality of the evidence before you. "The person that ignores data is the one that's surprised."
VRBPAC's Dr Hayley Gans notes she's struck by FDA asking committee to look at "totality of data when there are several key points that we're lacking right now," including very strong safety data
VRBPAC’s @DrPaulOffit speaks for first time & brings up age delineation: “While I would probably support a three-dose recommendation for those over 60 or 65, I really have trouble supporting this as written for anyone greater than or equal to 16.” 
Quick break to go on @CNBCClosingBell lmk what I miss!
VRBPAC's Dr Mark Sawyer says he'd be in favor of approving booster bc we're likely to need it for some of the population, and myocarditis safety data on booster isn't likely to be available until it's used extensively; says he hopes CDC rolls it out gradually
VRBPAC's Dr Jay Portnoy says he's "strongly in favor of approving this vaccine"
And now the committee is going to take an initial vote on approving booster for 16+ ... but likely just to get that official tally & then continue discussion possibly about different age group
And now the committee is going to take an initial vote on approving booster for 16+ ... but likely just to get that official tally & then continue discussion possibly about different age group
Pfizer gets one more chance to speak before going to vote - notes we'll only get sufficient information on myocarditis in pharmacovigilance data (ie when it's made more broadly available), and emphasizes "balance of evidence supports a broad recommendation"
VRBPAC voting now on 16+ recommendation for Pfizer booster (but this likely won't be end of meeting/or even necessarily final vote as some may want to change the age)
FDA advisory committee vote on whether safety & effectiveness data from Pfizer trial support approval of Comirnaty booster dose at least 6 months from primary series in people 16+
Yes: 3
No: 16
Abstain: 0
Yes: 3
No: 16
Abstain: 0
FDA VRBPAC members now discussing which ages or other groups they’d support #covid19 booster for - some say 50, some 60, others include people with comorbidities, and healthcare workers.
Likelihood of FDA approving a booster broadly and leaving clinical recs to CDC ACIP?
Likelihood of FDA approving a booster broadly and leaving clinical recs to CDC ACIP?
FDA clarifies there was an extra vote and the tally is actually 16-2 against a Pfizer booster for 16+.
Now they’re preparing language for a new voting question. Dr Marks proposes 65+ & those at high risk due to occupational exposure & underlying disease
Now they’re preparing language for a new voting question. Dr Marks proposes 65+ & those at high risk due to occupational exposure & underlying disease
Advisory committee meeting back from break. FDA's Dr Doran Fink says they're going to have a new voting question, but they're changing to Emergency Use Authorization instead of supplement to full approval
NEW voting Q for FDA advisory panel:
Based on totality of scientific evidence available... do known and potential benefits outweigh known and potential risks of Pfizer/BioNTech #covid19 vaccine booster at least 6 months out for use in:
-65+
-those at high risk of severe Covid
Based on totality of scientific evidence available... do known and potential benefits outweigh known and potential risks of Pfizer/BioNTech #covid19 vaccine booster at least 6 months out for use in:
-65+
-those at high risk of severe Covid
On new vote, for EUA for Pfizer booster for people 65+ and those at high risk of severe Covid:
Yes: 18
No: 0
Abstain: 0
Yes: 18
No: 0
Abstain: 0
FDA then asked expert panel whether healthcare workers or others at high risk for occupational exposure be included in Pfizer/BioNTech booster EUA
Yes: 18
No: 0
Abstain: 0
So recommendation is for 65+, those at high-risk of severe disease, and those with job exposure risk
Yes: 18
No: 0
Abstain: 0
So recommendation is for 65+, those at high-risk of severe disease, and those with job exposure risk
CDC's Dr Amanda Cohn notes these groups are the ones that got vaccinated earliest
And she says she hopes VRBPAC will meet again when there's more data to evaluate boosters in younger age groups
And she says she hopes VRBPAC will meet again when there's more data to evaluate boosters in younger age groups
And that's a wrap
See you back here Wed-Thurs, Sept 22-23, for CDC's advisory cmte meeting on boosters
(And before then, we await FDA's final decision - to accept recommendation or go its own way; this seemed very collaborative so would be surprised if FDA does something else)
See you back here Wed-Thurs, Sept 22-23, for CDC's advisory cmte meeting on boosters
(And before then, we await FDA's final decision - to accept recommendation or go its own way; this seemed very collaborative so would be surprised if FDA does something else)
• • •
Missing some Tweet in this thread? You can try to
force a refresh