An important new study looks at how COVID vaccines impacts symptoms in #LongCovid patients. @thitran3’s team used data from ComPaRe long COVID cohort to emulate a target trial (1:1 matched vax:unvax) measuring outcome at 120 days after baseline. (1/)
papers.ssrn.com/sol3/papers.cf…
papers.ssrn.com/sol3/papers.cf…
The study found that the rate of complete remission from long COVID symptoms doubled in vaccinated patients compared to unvaccinated long COVID patients. Wow, vaccines appear to be helping long haulers with recovery 👏🏼 (2/) 
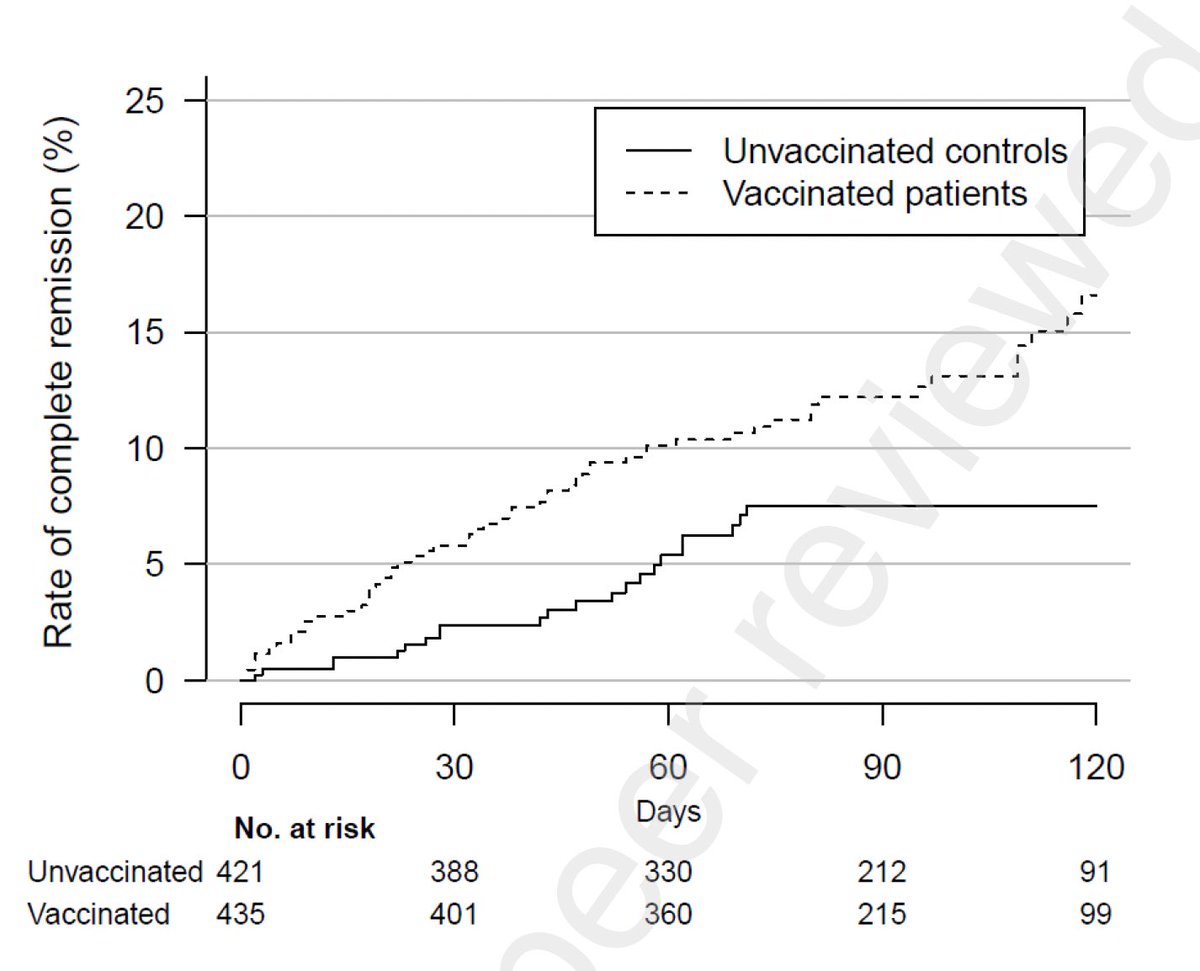
In addition, disease impact of long covid on patients’ lives were significantly reduced (symptoms improved) in vax group (long COVID IT score of 24.3) compared to unvax group (IT score of 27.6). (3/) 

What about severe adverse effects of vaccines in long haulers? Of the 455 long covid patients who received vaccines, 2 led to hospitalization, 2 led to ER visit, 13 had relapse of long covid symptoms. (4/) 

Overall, this study adds to growing evidence that vaccines can improve symptoms and lessen the disease impact in #longCOVID.
What is the evidence? An important patient survey from @LongCovidSOS showed impact of vaccines on long covid symptoms. (5/)
…e-4609-b25f-6f3d1497c4cf.filesusr.com/ugd/8bd4fe_a33…
What is the evidence? An important patient survey from @LongCovidSOS showed impact of vaccines on long covid symptoms. (5/)
…e-4609-b25f-6f3d1497c4cf.filesusr.com/ugd/8bd4fe_a33…

A prospective observational study by @gushamilton team showed small overall improvement in #longCOVID symptoms in vaccinated patients. (6/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
With input from @Survivor_Corps and @patientled, we are studying the impact of COVID vaccines on #longCOVID symptoms and correlating the changes in the immune responses to symptom changes. Led by @DaisySMassey @hmkyale (7/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
We hope to understand how vaccines are helping some long haulers and not others. Understanding the pathophysiology of #longCOVID is direly needed to develop diagnostic tools and therapy. Thank you @thitran3 et al for this important study 🙏🏼 (end)
• • •
Missing some Tweet in this thread? You can try to
force a refresh