The 1st randomized trial of a booster (3rd) shot in 10,000 people, placebo-controlled, shows 95.6% efficacy, with 5 cases (Pfizer vaccine) vs 109 in placebo group, Delta variant, broad benefit across age groups ft.com/content/d4e58d… 

Until now there were only vaccine effectiveness reports without randomization, without a placebo control group, and multiple potential confounding factors. That’s what makes this trial report significant proof of benefit of booster shots, at least for this vaccine regimen.
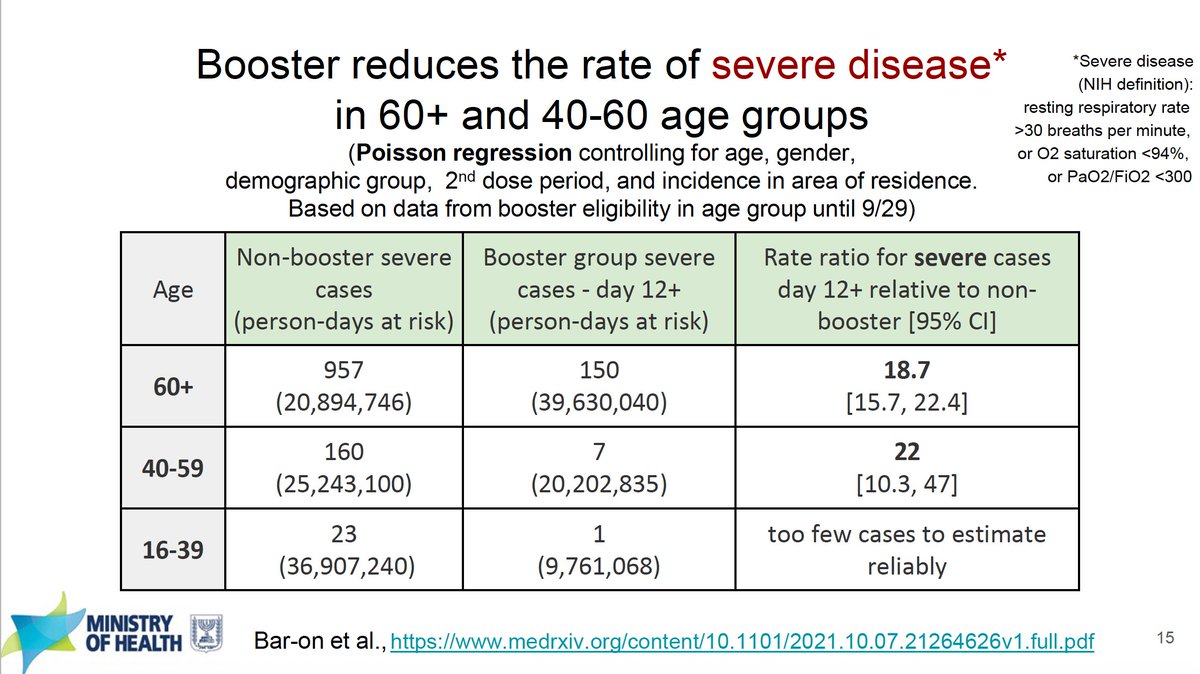
Back in April I was totally against booster shots due to lack of evidence of attrition of effectiveness and obvious interest of the companies to promote them. Now the data are abundant and IMO definitive for their benefit. Age 40+ vs severe disease.
https://twitter.com/erictopol/status/1383130179942322181?lang=en
The Israeli researchers and @IsraelMOH have done extraordinary work of rapidly accruing and publishing their unique data, teaching us about the impact of vaccination and fading immunity. They've not been adequately recognized for their contributions to date.
• • •
Missing some Tweet in this thread? You can try to
force a refresh