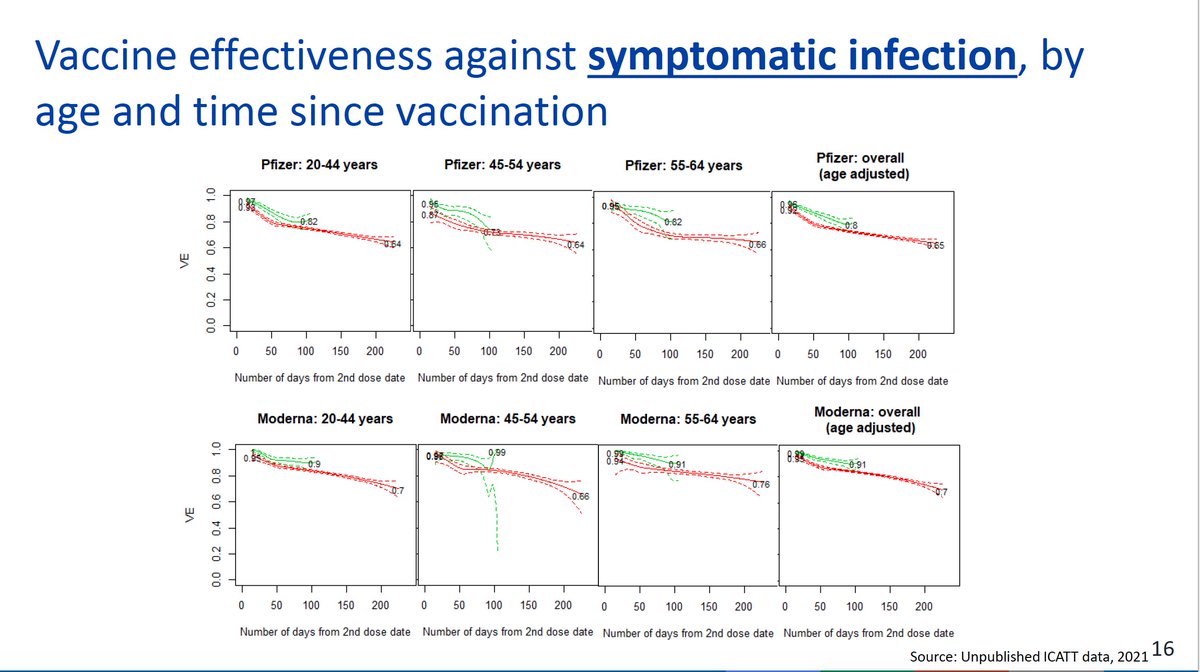
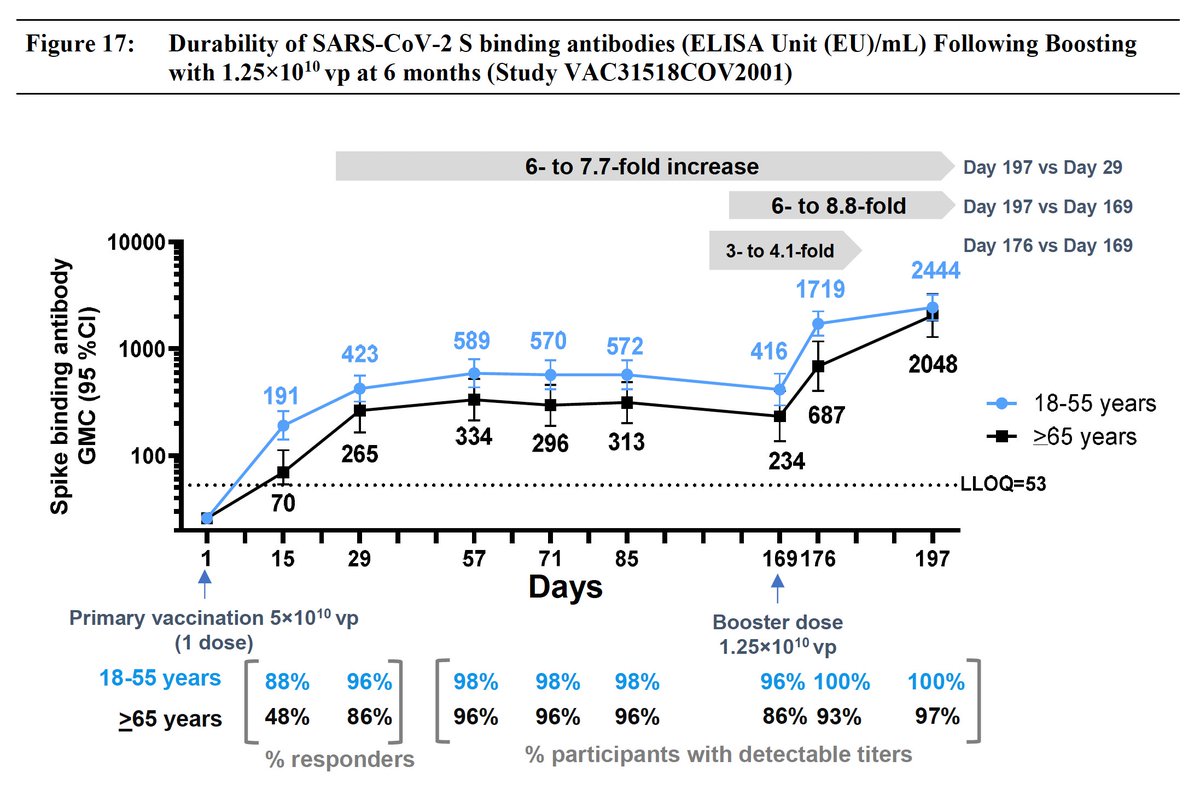
What data does @moderna_tx have to support its 50 ug booster dose? @US_FDA briefing documents provide immune response data across age groups but no evidence of restoration of clinical effectiveness fda.gov/media/152953/d… 

The same amended criteria given for Pfizer's emergency authorization are what Moderna is requesting. This will be an interesting VRBPAC meeting that essentially leverages effectiveness data from another vaccine (which I support) vs purists who will claim clinicaldata are lacking 

The J&J booster data for FDA review this week, like Moderna, has evidence for the immune response but without clinical effectiveness
fda.gov/media/152954/d…

fda.gov/media/152954/d…

• • •
Missing some Tweet in this thread? You can try to
force a refresh